The truth about vaccines
Leveraging Biotech's Drug Discovery Expertise For Neglected Diseases
Creating breakthrough drugs, diagnostics and vaccines that could roll back malaria, tuberculosis (TB) and other 'neglected' infectious diseases requires a long-term investment in product discovery. Calls for new strategies to develop such medicines have largely focused on recruiting discovery resources from the public sector and large pharmaceutical companies1,2. But shouldn't the biotech industry play a role? Today, biotech companies invent most of the new medicines entering the pipeline for such diseases as cancer, cardiovascular disease and Alzheimer's, as well as infectious diseases. It's been unclear, however, how their discovery platforms and business models are relevant to neglected diseases.
Biotech business and neglected diseases
With $400 billion of equity investment, more than 4,300 companies and over 270 approved products3 (http://bio.Org/healthcare/), the biotech industry represents a rich source of expertise and resources that could be deployed to tackle neglected disease. What makes neglected diseases neglected, however, is that few patients can afford treatment and the private sector lacks inducements to invest in R&D for diseases prevalent in poorer countries. Indeed, it's frequently noted that between 1975 and 2004, only 21 out of 1,556 drugs approved by regulatory authorities were for neglected diseases4,5.
But the global health landscape is changing rapidly. The science of many tropical pathogens has benefited from over 30 years of solid biochemical research supplemented by complete genome sequencing. Visionary and deep-pocketed donors, such as the Bill & Melinda Gates Foundation, are supporting a new generation of healthcare products for the neglected diseases that burden the developing world. International partnerships, such as The Global Fund (Geneva) and the GAVI Alliance (Geneva) are creating sustained purchasing power for drugs and vaccines for the developing world. A bevy of other nonprofit, public-private partnerships that champion product procurement, distribution and R&D are generating new hope for the hundreds of millions at risk.
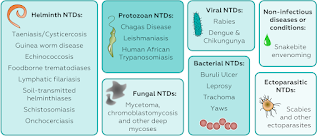
Despite the past decade's progress, the pipeline of new products is insufficient to control, much less eliminate, the most devastating neglected diseases. There is a dearth of vaccines; few diagnostics are designed for use in resource-poor settings; and many of the available drugs are marginally effective, or, worse, toxic. For want of adequate medicines, nearly two million die from TB causes per year and malaria exacts a similar toll. Hundreds of millions of people are debilitated by a staggering array of protozoan and helminthic parasites. As many as one in five children in the poorest parts of sub-Saharan Africa don't survive to age five.
These are urgent problems, and to help solve them, we need to apply the discovery platforms that fuel the majority of today's biopharmaceutical pipeline. At BIO Ventures for Global Health (BVGH; Washington, DC, USA), we believe that innovative biotech companies can play the same pivotal role in neglected diseases that they've played in creating therapeutics, vaccines and diagnostics for the industrialized world.
We distinguish the biotech industry from pharma as follows: the pharmaceutical industry evolved from chemical companies founded in the late 1800s and early 1900s, and today is dominated by some 17 large, innovative companies with a global reach; in contrast, the biotech industry is much younger and entrepreneurial, springing up to take advantage of recombinant DNA technology developed in the mid-1970s. Of the hundreds of companies worldwide, most grew out of molecular biology discoveries from academic research laboratories, received initial support from venture capitalists and are focused on innovation. Thirty years or so after the industry's inception, they range from small startups with a handful of employees and a bright idea but no products or revenues (by far the majority) to a few large companies that aren't easy to distinguish from big pharma in terms of overall size, breadth of pipeline and revenues.
A crucial bridge from familiar diseases of the developed world to tropical pathogens lies at the level of molecular targets, particularly in drug discovery. Kinases, proteases, ion channels, cytoskeletal proteins and many other familiar drug discovery targets have homologs in neglected disease pathogens. Small-molecule discovery platforms developed by biotech should be amenable to creating new hits and lead compounds for validated targets in the neglected disease pathogens, despite the unique features of infection and persistence. Although we do not explore them in this article, similar opportunities to translate core biotech capabilities exist in vaccines and diagnostics, particularly antigen and biomarker discovery.
The innovation gap in neglected-disease drug discovery
Recently, BVGH published Closing the Global Health Innovation Gap, the first in a planned series of reports that assesses how the biotech industry's diverse technology platforms and expertise could be applied to neglected-disease product development. For the purposes of our study, we were most interested in examining small and mid-size companies (fewer than 200 employees and <$1 billion in market capitalization). Although vaccines are among the most cost-effective tools for preventing disease, and diagnostics are vital to making effective clinical decisions, we focused our analysis on the need for new drugs because there are major unmet needs for therapy and the vast majority of biotech companies work on drugs. Although many neglected diseases could benefit from new therapeutic options, we selected malaria, TB and human African trypanosomiasis (HAT) for three reasons. First, drug target identification has been facilitated by the availability of the fully sequenced and annotated genomes for Plasmodium falciparum, Mycobacterium tuberculosis and Trypanosoma brucei, the etiological agents responsible for the most deadly form of malaria, TB and HAT, respectively. Second, genetic tools have been developed for these agents to validate potential drug targets. And third, animal models of infection exist, making it possible to test the ability of compounds to kill pathogens in vivo. Thus, essential drug discovery tools are in place.
In addition, the public sector has launched multiple R&D initiatives for these neglected diseases, including academic drug discovery centers and product development partnerships (PDPs) (Box 1 and Table 1). Leading PDPs include the Medicines for Malaria Venture (MMV; Geneva), the Drugs for Neglected Diseases initiative (DNDi; Geneva), the Institute for One World Health (iOWH; San Francisco) and the Global Alliance for TB Drug Development (TB Alliance; New York). These nonprofits act as virtual drug development enterprises; they consist of lean organizations focused on project management and coordinate outsourced R&D efforts through partnerships with industry, contract research organizations and academic laboratories.
Table 1 Selected PDPs involved in drug, diagnostic and vaccine development for neglected diseasesThe therapeutics-focused PDPs have set their sights high, aiming to create a robust pipeline with new products registered every few years. Initially, they chose to concentrate on known chemical entities to expedite clinical development, resulting in important, but incremental, innovation. To succeed with this model, they acquired candidates with promising data in target diseases or created new formulations or fixed-dose combinations of existing drugs. This approach has already borne fruit with the registration of three products for malaria7,8,9 and one for visceral leishmaniasis10.
As today's first-generation candidates advance to clinical success and registration, or clinical failure and abandonment, they will need to be replaced with new compounds that have increased efficacy, are more easily administered, are stable in harsh environments and yet remain inexpensive to produce and affordable to purchase. The high attrition rates inherent in early-stage drug discovery require that hundreds of screening projects be pursued simultaneously to generate a sufficient number of investigational new drugs to yield a single approved drug. The PDPs, a few companies and several universities, research institutes and academia-led consortia have taken on this challenge and initiated drug discovery programs11,12. But to provide enough compounds to overcome the harsh statistical realities of drug attrition rates, more must be done. Industry-driven programs for a disease with an established market are often spread across many companies, and their scope is many times the size of today's discovery and preclinical pipelines for malaria, TB and HAT (Fig. 1). This disparity—which we refer to as the 'innovation gap'—means that too few discovery programs exist to ensure a steady stream of approved treatments. The innovation gap is not unique to malaria, TB and HAT; on the contrary, it is even more pronounced for such diseases as Chagas and lymphatic filariasis, which receive considerably less attention and resources than either malaria or TB.
Figure 1: Expected project attrition rate versus the current neglected disease drug pipelines.On average, for every 100 projects entering the screening phase of drug discovery, 1.3 products will successfully reach the market 12–14 years later. The total number of projects in each phase of discovery and development as of July 2008 were quantified for malaria, TB and HAT. Assuming standard attrition rates apply, there are too few projects in discovery to ensure that new products will emerge from the pipeline. (Sources: MMV, DNDi, TB Alliance, BVGH)
New approaches are needed to provide incentives for biotech companies to invest in new medicines for neglected diseases where patients have little purchasing power. Credit: James Yang/© Images.Com/Corbis
Greater investment in discovery is critical to sustaining a pipeline of new medicines for neglected diseases. Where will it come from? One answer, we believe, lies in leveraging proven discovery platforms that reside in biotech companies.
Target practice: there's more than one bull's-eye
Biotech companies are often characterized in terms of the diseases they address. Many, however, are able to take on multiple diseases because their choice of specialized molecular targets may underlie mechanisms of several diseases. Examples include proteases, ion channels and kinases. In practical terms, this means that companies with drug discovery technologies specializing in a particular target class may be able to expand their discovery programs into multiple diseases. For example, proteases are studied for HIV, cardiovascular disease and hepatitis C virus; ion channels are pursued for pain, hypertension and epilepsy; kinases are involved in multiple types of cancer; and phosphodiesterases are implicated in pulmonary arterial hypertension and erectile dysfunction.
If targets related to those being pursued for conditions like cancer and cardiovascular disease are validated in neglected disease pathogens, biotech companies are in an advantageous position to extend their assets and expertise. As summarized in Figure 2 and explored in more depth in Box 2, significant target class overlap does exist between diseases commonly pursued by biotech and malaria, TB and HAT.
Figure 2: Targets are transferable across diseases.Many drug targets that have been validated in diseases with a commercial market have also been validated in malaria, TB and/or HAT. This means, theoretically, that industry's existing drug discovery assets and expertise could be leveraged for neglected diseases.
Target overlap notwithstanding, some might argue that big pharma is far better positioned than biotech companies to help overcome the innovation gap. Whereas pharmaceutical companies are highly profitable and able to afford in-kind contributions of R&D personnel and resources, biotech companies lack philanthropy budgets. Their business models and investor expectations may make it hard for them to incur the opportunity cost of dedicating scientists and managers to projects that don't promise a high return, even if outside sources provided complete full-time equivalent (FTE) funding.
Others may not see how biotech fits because of confusion about the industry's original focus on macromolecules—proteins or antibodies made by recombinant DNA technologies. Although the biotech sector's early breakthroughs were protein drugs that treat chronic diseases and these products continue to generate the largest revenues3, over the past 15 years the industry has been transformed. Today, most biotech companies and small specialty pharma companies develop small molecules that have the potential to be inexpensively synthesized, formulated for oral delivery and targeted to infectious diseases.
Taking advantage of new information and technologies—particularly fully sequenced genomes, advances in high-throughput screening and purpose-built compound libraries—this new crop of companies leads innovation of small-molecule drugs, previously the domain of big pharma. From 1998 to 2003, when the biotech effort in chemistry was still expanding, just over 25% of small molecules approved by the US Food and Drug Administration (FDA) originated in biotech13. According to the business intelligence firm LEK Consulting (Boston, MA), as of January 2007, two-thirds of small-molecule drugs in the development pipeline had biotech origins.
Indeed, the fundamental argument for biotech companies to play an integral role in alleviating neglected diseases is that they have become the engines of innovation for pharmaceutical development worldwide. Despite substantial internal investments in R&D and repeated restructuring to create more 'entrepreneurial' R&D teams, large pharmaceutical companies are increasingly sourcing technology and products from the biotech sector. Whereas academic institutions excel in basic research and pharma companies have the massive infrastructure required for late-stage trials, manufacturing and distribution, biotech companies are the principal inventors of truly novel medicines. Moreover, they have the flexibility and entrepreneurial drive to take on high-risk opportunities.
Identifying sharpshooters: companies poised for neglected disease R&D
Potentially hundreds of biotech companies can contribute to global health. But for the purposes of our Closing the Global Health Innovation Gap report, we defined a subset of broadly capable small-molecule discovery companies that could potentially contribute proven platform capabilities to new targets. We chose to limit our analysis to the over 1,500 North American and European companies backed by institutional investment that are most likely to have the wherewithal to support R&D efforts.
Our criteria were to select companies that (i) focus on small-molecule drugs as opposed to therapeutic proteins; (ii) have significant discovery capabilities; (iii) have a track record of taking new compounds into the clinic; and (iv) have sufficient size and scale to take on new programs. We arrived at a list of ∼120 companies that could be logical first prospects for neglected-disease drug discovery partnerships.
We looked at these companies by target focus (Fig. 3). Many of the target classes they are pursuing have been validated as targets in neglected diseases (Fig. 2). Through interviews with scientists and senior executives at a number of companies, we found that they typically possess three key technology assets built around target classes: specialized high-throughput screening assays, comparative structural information for multiple targets and highly refined compound libraries.
Figure 3: The drug target focus of leading biotech companies.The product portfolios of 47 companies (a subset of the 120 identified as logical prospects for neglected-disease drug discovery partnerships) were broken down on the basis of target class. Target classes representing ≥50% of a company's portfolio, 10–50% and <10% are indicated. GPCR, G protein–couple receptor. (Source: PharmaProjects)
At least three approaches could be envisioned to employ their infrastructure and proprietary technologies to attack drug discovery for neglected diseases. In the first, high-throughput screening could be used against a conserved target. Certain pathogen targets have already been validated for which biological function is comparable to that of a human protein of the same target class. Here, a company could apply its high-throughput screening assay format to identify hits and lead compounds, and its target-focused compound library for screening, improving the odds of identifying hits and leads that could be quickly confirmed to be structure dependent and dose responsive.
A second approach would be high-throughput screening against a conserved target class that is known to be essential for viability or virulence in a pathogen, but for which specific targets have yet to be validated. Using a focused compound library, whole-cell screening could be performed to identify compound hits that kill the target pathogen. Chemogenomic approaches could then be used to identify the specific protein in the target class bound by the hit compound. With the pathogen protein target in hand, leads could be identified and further optimized.
Finally, high-throughput screening could be used against a novel target class—certain biologically validated targets in the pathogen that do not correspond to families that companies have already explored. For example, genes of the apicoplast (an ancient, nonphotosynthetic plastid) in P. Falciparum are required for survival, but have no homologs in mammalian cells. Yet there are still ways to use biotech assets against these targets. Companies with generalized discovery platforms have compound libraries that emphasize chemical diversity and drug-like properties over other considerations and that could be screened against novel target classes.
Drug discovery, of course, is not an end to itself, but rather one step in the process of creating a new product. For diseases with a developed-world market, a continuum of collaborating players has evolved to ensure that products move efficiently from the research laboratory to the clinic to the patient's bedside (Fig. 4). Commercially viable ideas and inventions created in research universities and other institutions are typically licensed to biotech innovators, who translate basic science into nascent products. With a promising candidate in hand, biotech companies partner with pharma to carry out clinical and regulatory activities.
Figure 4: A continuum of players must be assembled to move from basic research to product registration.Neglected disease R&D lacks the continuum of players and handoffs that have evolved for developed-world diseases with a paying market.
For neglected diseases, the path leading from basic research through product discovery, development and registration is still being formed. Few companies have either the neglected disease expertise to initiate a program or the resources to move a promising compound into clinical development. Even so, companies can acquire disease expertise by partnering with the many academic groups that are pursuing neglected disease research and have developed tools and technologies relevant to drug discovery. Likewise, the advent of PDPs and the presence of several large pharmaceutical companies that have made a commitment to global health means that there are now potential partners to take products into clinical trials, through product registration and into the field.
Building on biotech: setting companies' sights on neglected diseases
Historically, biotech companies haven't shied away from taking on orphan diseases that affect small patient populations. So how do we recruit them to take on neglected diseases that affect hundreds or thousands of times as many people? Simply put, companies need a menu of incentives and up-front R&D funding that will allow them to deploy their scarce resources on these new opportunities.
Biotech companies that have already moved into neglected-disease product development tend to fall into two groups: smaller companies, for whom public-sector grant funding is meaningful, and large, well-established companies whose motivations for participating are similar to those of big pharma—namely, investments in global health can bring benefits ranging from improved public image and workforce motivation to a potential competitive advantage in emerging markets. Fewer of the mid-tier, institutionally backed drug discovery companies that comprise most of our top 120 focus companies have launched global health projects. These innovators have attracted substantial equity capital, but still don't have the financial leeway to be philanthropic. Thus, they have been slower to embrace neglected-disease drug discovery than their larger or smaller counterparts. Nonetheless, we've counted roughly 100 companies who have global health R&D projects under way. Today's early adopters offer clues to the strategic and business motives that drive their interest.
One motivation to get involved is the chance to prove technology platforms. Working on a neglected disease can be a way to access nondilutive financing from funding agencies and/or PDPs. This funding, in turn, can be used to support proof-of-concept experiments, proving the utility of a company's technology platform14. For example, by leveraging their not-for-profit partnership with the Gates Foundation and iOWH on the production of semi-synthetic artemisinin (an anti-malarial), Amyris Biotechnologies (Emeryville, CA, USA) was able to attract substantial venture capital investment to apply their platform to biofuels. Likewise, Anacor Pharmaceuticals (Palo Alto, CA, USA) recently entered a collaborative agreement with DNDi to test their proprietary boron-based chemistry platform on HAT15. The deal provides Anacor with an opportunity to learn valuable information about its compounds, which they can apply to internal, for-profit discovery programs.
Another motivation is the opportunity to make a major contribution to global health. Carrying out R&D on neglected diseases can be a powerful incentive for scientists. Many companies reported to us that staff scientists have practically beaten down their CEOs' doors to take part in projects related to neglected diseases. Initiating such projects can be a way for a company to attract and retain talented scientists.
And a third motivation to participate in neglected disease research is to sustain an underutilized discovery platform, which often is found in companies devoting all their resources to advanced compounds in late stages of development. To sustain their discovery platform, several companies have been willing to engage in screening programs in neglected diseases on a no-profit/no-loss basis.
Nonetheless, these motives alone can't overcome the dearth of financial incentives or foster the industry investment needed to close the innovation gap. For most companies, the realities of the marketplace—the relentless bottom line—demand that they focus on established developed world markets. To attract the most capable innovators to neglected-disease product development, substantial new 'push' as well as 'pull' financial incentives (Box 3) and research funding are needed.
Today, most global health R&D funding is directed toward development rather than discovery. To close the innovation gap, we must work not only to grow the funding 'pie,' but also to increase the proportion of funding allocated to discovery. For example, MMV spent only ∼$15 million16 on discovery in 2007, roughly comparable to the amount a single biotech company spends on discovery each year. Generating a single candidate for clinical development costs ∼$20 million. With anti-infective attrition rates near 84%, at least six clinical candidates, at a cost of $120 million, might be needed to generate a single approved drug2. To ensure a new drug emerges from the pipeline every few years will require more still. And this is just for malaria. TB, HAT and any of the other five diseases, identified by the World Health Organization's (Geneva) Special Program for Research and Training in Tropical Diseases as desperately needing new drugs, will require similar sums2.
To help companies with a strong desire to initiate global health R&D, we need to overcome barriers that keep them from transforming their ideas and ambition into action. This will entail educating companies about the opportunities to apply their technologies, and pointing out how markets are being created and how incentives can be exploited. We'll also need to facilitate partnering and direct funding for the most promising programs. Companies will need to be connected with academic experts and physicians who understand the biology of neglected diseases, with global health officials who have established target product profiles, and with PDPs or large pharmaceutical companies that have experience conducting clinical trials in the developing world, navigating the regulatory system and ensuring products are delivered to patients. If these goals can be achieved, we'll arrive at our ultimate target that much faster—the day when neglected diseases are neglected no longer.
Box 1: Product development partnershipsProduct development partnerships (PDPs) are not-for-profit companies focused on product development for neglected diseases. Over a dozen of these innovative organizations have arisen to fill the void in R&D for neglected diseases left by the private sector. PDPs manage new-product portfolios, drive preclinical and clinical development, make 'go/no-go' decisions and coordinate clinical trials in developing countries. Many PDPs are 'virtual', with lean organizations focused on project management rather than developing products directly. They manage R&D activities through partnerships with industry, and contract research organizations and academic groups. Other PDPs do conduct R&D in their own laboratories and manage major programs. Several PDPs mix these models. PDPs have done a remarkable job of assembling skilled teams, raising money (in some cases hundreds of millions of dollars in grants) and building diverse portfolios. Leading PDPs are listed in Table 1.
Box 2: Target classes with crossover to neglected diseasesAs part of our study, we identified several target classes currently under investigation by the biotech sector for diseases common in the Western hemisphere that are also relevant to R&D on neglected diseases. Examples include proteases, cyclic phosphodiesterases and kinases. Though under development for diseases common in the Western hemisphere, these targets are also relevant to R&D on neglected diseases.
Proteases cleave peptide bonds, breaking down proteins into peptide chains or single amino acids. Drugs on the market that target proteases include Novartis' (Basel) Tekturna (aliskiren), which was developed by Speedel Pharma (Basel) for the treatment of hypertension, as well as Pfizer's (New York) Viracept (nelfinavir; licensed from Agouron Pharmaceuticals, La Jolla, CA, USA) and London-based GlaxoSmithKline's Lexiva (fosamprenavir; originally developed by Vertex Pharmaceuticals, Cambridge, MA, USA), both of which are therapies against HIV. Clinical trials are currently underway to test the efficacy of various protease inhibitors as therapeutics for hepatitis C virus and osteoporosis. But proteases might also be targets for malarial drug development. Inside a red blood cell, for example, P. Falciparum derives energy and nutrients by breaking down hemoglobin, a process that requires the activity of several parasite proteases17. Among them, a cysteine protease subfamily known as falcipains has been validated and pursued as a collaboration between MMV and GlaxoSmithKline (London). Other proteases continue to be explored as targets in P. Falciparum and have been validated in M. Tuberculosis and T. Brucei.
Cyclic nucleotide phosphodiesterases (PDEs) break the phosphodiester bond found in cyclic nucleotides involved in eukaryotic signal transduction. Inhibitors of the PDE-5 subclass are used as vasodilators to treat pulmonary arterial hypertension (e.G., Revatio, sildenafil; Pfizer, New York) and erectile dysfunction (e.G., Cialis, tadafil; Lilly, Indianapolis). New inhibitors of different PDEs are being pursued for conditions ranging from depression to inflammation. The T. Brucei genome encodes five PDEs and cannot survive in the absence of two of them, TbrPDEB1 and TbrPDEB2 (ref. 18). Because the catalytic sites of TbrPDEB1 and TbrPDEB2 are nearly identical, it may be possible to design a single inhibitor of both enzymes. PDEs are also considered validated targets in P. Falciparum19.
Finally, protein kinases, which transfer a phosphate group from an ATP molecule to a protein substrate, play fundamental roles in cell division and signal transduction, processes that often malfunction in cancerous cells. Kinase inhibitors hold enormous therapeutic potential for cancer. For example, Novartis' Gleevec (imatinib), an inhibitor of the protein kinase BCR-ABL, has been approved for use in chronic myeloid leukemia and gastrointestinal stromal tumors, and it is being pursued for treatment of a variety of additional cancers. Because of their essential role in cell proliferation, kinases may also be key targets in preventing replication of infectious pathogens. All of the genes predicted to encode kinases ('the kinome') in P. Falciparum and T. Brucei have been described20,21. For most of these genes, the biology remains to be worked out, and few have been genetically or chemically validated. Nonetheless, kinases involved in cell-cycle control have been chemically or genetically validated for both P. Falciparum (PfMrk)22,23 and T. Brucei (CRK3)24,25. PknG, a M. Tuberculosis kinase required for bacterial survival within the host macrophage, has also received attention as a new drug target26,27.
Box 3: Incentivizing global health product developmentHow can companies, particularly cash-strapped biotech firms, be convinced to invest in new medicines for neglected diseases when the patients themselves have no purchasing power? Patients' need for new therapies doesn't automatically translate into a market that can support development of a product. As a response to this problem, governments and donor organizations have begun to design financial incentives to spur R&D in neglected diseases.
Economists talk about 'push' and 'pull' incentives; the former provide up-front funding that reduces risks and mitigates the cost of R&D, whereas pull mechanisms enhance market opportunities by providing downstream rewards for success. The most familiar push incentives are research grants and full-time employee (FTE) funding from contracts with large pharma companies or government agencies.
Pull incentives include forward contracts, royalties and prizes, and are favored by some because they only reward success. The rewards of pull incentives must be substantial enough to compensate an innovator for taking on the risk of failure. Although pull incentives for neglected diseases are in their infancy, several important experiments are already under way.
Advanced Market Commitments (AMCs) guarantee a market for new vaccines in developing countries (http://www.Vaccineamc.Org/). A $1.5 billion pilot AMC for pneumococcal vaccines, sponsored by five industrialized countries and the Bill & Melinda Gates Foundation, will be officially launched this year.
Priority Review Vouchers (PRVs), created by the FDA Amendments Act of 2007, provide any organization that gains FDA approval for a novel drug or vaccine to treat or prevent a neglected disease a 'voucher' for priority review from the FDA for a future product28. The voucher can be traded or sold; its value is proportional to the accelerated market entry for a prospective 'blockbuster' product.
Prizes for specified technological advances are under discussion, such as the X-Prize for tuberculosis diagnostics29.
In the process of perfecting push-and-pull incentives, many issues are being hashed out. But one thing's for certain—there is no 'one size fits all' solution. Push funding, for example, has been instrumental in building current global health pipelines, but it isn't a sufficient incentive for all companies because it doesn't address the problem of opportunity cost and doesn't guarantee a market for a finished product. AMCs and PRVs may address those issues for larger companies with long time horizons, but may be less effective for smaller biotech companies whose financing time horizons are typically less than two years. For those organizations, nearer-term incentives may be needed.
One idea that combines aspects of both push and pull is milestone-based incentives that reward intermediate stages of development such as major safety and proof-of-concept clinical data. Much work remains to be done, however, to explore whether such incentives would be attractive to both industry and donors.
DeepMind Uses AI To Tackle Neglected Deadly Diseases
The technology will study Chagas disease, which is transmitted by assassin bugs
Artificial intelligence is to be used to tackle the most deadly parasitic diseases in the developing world, tech company DeepMind has announced.
The London-based Alphabet-owned lab will work with the Drugs for Neglected Diseases initiative (DNDI) to treat Chagas disease and Leishmaniasis.
Scientists spend years in laboratories mapping protein structures.
But last year, DeepMind's AlphaFold program was able to achieve the same accuracy in a matter of days.
Many diseases are linked to the roles of proteins in:
And knowing the 3D structure of a protein is important in developing treatments for, among others, cancer, dementia and infectious diseases.
A DeepMind model of a protein from the Legionnaire's disease bacteria (Casp-14)
"We've been excited by the potential for this technology to help fill in some of the gaps in our understanding of biology and accelerate scientific research to enable new, effective treatments for diseases," DeepMind AI-for-science head Pushmeet Kohli said.
And the company wanted to focus on "underserved" and "neglected" areas.
"We hope AlphaFold will have a real-world impact on our understanding of disease and drug discovery for communities who are in great need of treatments," he said.
Serious side-effects
Chagas disease and Leishmaniasis affect up to 23 million people worldwide.
And repurposing existing drugs has proven ineffective.
"Patients affected by neglected diseases like Leishmaniasis and Chagas disease rely on outdated treatments that are sometimes toxic, have serious side-effects, and are often not fit for purpose," DNDi discovery lead Ben Perry said.
And he hopes the partnership will lead to the discovery of drugs that will be easy to take orally.
"AI can be a game-changer - by predicting protein structures for previously unsolvable protein structures, AlphaFold opens new research horizons," Mr Perry said.
"It is heartening to see powerful cutting-edge drug discovery technologies enabling work on some of the world's most neglected diseases."
'Great hope'
Prof Dame Janet Thornton, of the European Bioinformatics Institute, told BBC News: "Most new drugs in recent years have been developed using protein-structural data as one part of the process.
"There are, however, many other aspects which need to be taken into account, which, due to lack of data, may not be amenable to AI approaches."
But the predictions would be "particularly valuable" for pathogens with unknown protein structures, including some neglected diseases.
"Developing new AI approaches for designing such drugs is a new challenge but one to which the new AI techniques can be applied and this holds out great hope for the future," Dame Janet added.
Free access
And as a result, some partners withdrew from its Streams app, which helps doctors and nurses in some NHS hospitals monitor patients with kidney conditions.
But last year, the AI program was used to map some Sars-Cov-2 virus proteins.
DeepMind will also be publishing a peer-reviewed paper detailing the workings of its system, providing free access to AlphaFold for the scientific community.
And it plans to collaborate on tackling other diseases in future.
One of biology's biggest mysteries 'largely solved'
Google swallows DeepMind Health
Google health app takeover sparks concerns
Center For Drug Discovery
Content
The Center for Drug Discovery is a strategic center and resource for investigators at Baylor College of Medicine and beyond. The CDD quickly identifies and advances small molecules to clinical trials, with the goal of developing cost-effective, novel drug and treatment options. The CDD is devoted to finding new cures for a wide range of diseases, including infectious diseases, neurological diseases, metabolic disorders, cancer, diabetes, cardiovascular diseases and inflammatory disorders. Its mission is to develop small molecule probes, preclinical candidates and drugs for researchers and clinicians in the Texas Medical Center and beyond. Though based at Baylor, CDD members include faculty at other institutions in the Texas Medical Center, most notably Texas Children's Hospital and The University of Texas MD Anderson Cancer Center.
The CDD bridges the gap between academic research and pharmaceutical discovery and provides researchers with an economically viable entry into early-stage drug discovery as well as enable the study and validation of protein targets and disease mechanisms. The CDD uses state-of-the-art equipment and methodologies that will yield better candidate compounds for drugs and experimental therapeutics.
The center employs techniques that sample more fully the theoretical total "chemical space" that comprises all possible chemical synthesis reaction products, resulting in compounds that more efficiently fill three-dimensional molecular space and better modulate the functions of specific target proteins. Chief among these methods are DNA-encoded chemistry technology and fragment-based library discovery that will lower the cost of drug discovery, speed up the process and bring new hope to finding cures for some of the most urgent diseases that afflict human populations.
To date, the CDD has produced libraries of compounds (now totaling over four billion compounds) that will translate into new leads, commercial opportunities, and licensure. The ultimate aim is to devise new therapies to alleviate a wide range of illnesses, including infectious disease, diabetes, heart disease, inflammatory disorders, cancer, neurodegeneration and other challenging conditions for which few effective treatments exist.

Comments
Post a Comment