Whole genome sequence-based characterisation of Shiga toxin ... - Nature.com
Abstract
Game meat is becoming increasingly popular but may be contaminated with pathogenic bacteria such as Shiga toxin-producing Escherichia coli (STEC). STEC cause gastrointestinal illnesses including diarrhoea, haemorrhagic colitis (HC), and the haemolytic uremic syndrome (HUS). The aim of this study was to assess the occurrence of STEC in 92 meat samples from chamois (n = 2), red deer (n = 27), roe deer (n = 38), and wild boar (n = 25), from Switzerland and other European countries. After enrichment, Shiga-toxin encoding genes (stx) were detected by PCR in 78 (84%) of the samples and STEC were isolated from 23 (25%) of the same samples. Nine different serotypes and eight different sequence types (STs) were found, with O146:H28 ST738 (n = 10) and O110:H31 ST812 (n = 5) predominating. None of the STEC belonged to the so-called top-five serogroups O26, O103, O111, O145, and O157. Subtyping of stx identified stx1c (n = 9), stx2a (n = 1), stx2b (n = 19), stx2e (n = 2), and stx2g (n = 1). Additional virulence factors (VFs) comprised ehx (n = 12), iha (n = 21), sta1 (n = 1), and subAB (n = 19). None of the isolates contained the eae gene. Twenty-one STEC contained VFs associated with extra-intestinal pathogenic E. coli (ExPEC). Overall, the pathogenic potential of STEC in game meat is moderate, though the isolation of one STEC strain carrying stx2a, and of STEC/ExPEC hybrids suggests a role of game meat as a potential source of STEC infections in humans. Therefore, detailed knowledge of the safe handling and preparation of game meat is needed to prevent foodborne infections.
Introduction
Shiga toxin-producing Escherichia coli (STEC) cause an estimated 2.8 million acute illnesses annually, representing one of the most common causes of gastrointestinal illness worldwide1. STEC may cause mild to severe non-bloody or bloody diarrhea (BD), haemorrhagic colitis (HC), and the life-threatening haemolytic uremic syndrome (HUS)2. STEC are characterized by two types of Shiga toxins encoded by stx1 and stx2, with four stx1 (stx1a, stx1c, stx1d, and stx1e) and 14 stx2 (stx2a-stx2m, and stx2o) subtypes described so far3,4,5,6. STEC harbouring stx2a and stx2d are understood to be associated with severe disease whereas STEC carrying stx2b and stx2e are typically linked to mild clinical symptoms or asymptomatic faecal carriage7,8. Other stx2 subtypes including stx2f, stx2g, stx2m and stx2o are infrequently identified in STEC from human samples, but stx2f-positive strains have been isolated from patients with HUS5. Furthermore, many STEC strains feature additional virulence genes encoding toxins and adherence factors such as astA (enteroaggregative E. coli heat-stable toxin 1), eae (adherence factor intimin), ehxA (enterohemolysin), iha (IrgA homolog adhesin), lpf (long polar fimbriae), and subAB (subtilase cytotoxin)9,10. Moreover, STEC may also exhibit virulence properties from other E. coli pathotypes such as enteroaggregative E. coli (EAEC) or extraintestinal pathogenic E. coli (ExPEC), for example the STEC/EAEC hybrid serotype O104:H4 that caused the major HUS outbreak in Germany in 201111,12, or the STEC/ExPEC hybrid serotype O80:H2 which has emerged in France and Switzerland as a serogroup causing HUS and bacteraemia13,14,15.
Although frequently linked to food borne outbreaks, the majority of STEC infections remain sporadic and are significantly associated with person-to-person transmission, contact with animals or their environment, and consuming undercooked or raw meat, in particular beef16,17,18.
Meat from wild game is gaining in popularity in many countries as it appeals to a growing demand for foods that are nutritious and serve as an alternative to conventional meat from intensive livestock production19. Despite the growing interest in meat from game animals, European legislation (Commission Regulation (EC) No. 2073/2005) does not specify hygiene criteria for raw wild game meat regarding STEC, and information on the prevalence and pathogenicity of STEC in this food category is limited20.
The aim of this study was therefore to assess the occurrence of STEC in meat samples of chamois (n = 2), red deer (n = 27), roe deer (n = 38), and wild boar (n = 25) originating from Switzerland and other European countries, and to analyse the STEC isolates for serotypes, multilocus sequence types, and virulence gene content, using a whole genome sequencing approach.
Results
Real-time screening for stx genes and isolation of STEC
Using real-time PCR screening identified stx1 and/or stx2 in 77 (84%) of the 92 game meat samples analysed in this study. Thereof, the majority (75 of 77 samples) contained stx2, alone or in combination with stx1. The positive samples included one of the two chamois, 24 of the 27 red deer, 36 of 38 roe deer, and 16 of 25 wild boar meat samples (Table 1).
STEC were isolated from 23 of the 77 stx-positive real-time PCR samples, corresponding to a recovery rate of 30% and an overall STEC prevalence of 25%. The roe deer meat samples W42, W96, W98, and W99 contained two distinct STEC isolates resulting in a total of 27 STEC available for further analysis (Table 1).
Serotypes, multilocus sequence types (MLST) and phylogenetic relationship
Overall, nine different serotypes were identified among the 27 STEC (Table 2). O146:H28 and O110:H31 were the predominant serotypes, accounting for 10 (37%), and 5 (19%) of all STEC isolates (Table 2). Eight sequence types (STs) were assigned among the 27 STEC, thereof, ST738 (n = 10) and ST812 (n = 5) were predominant. Isolates with the same serotype were assigned to the same sequence type, with the exceptions of one STEC O21:H21 (isolate B42 recovered from roe deer meat), and STEC O179:H8 (isolate B75-8 recovered from wild boar meat), which were both not assigned to any ST (Table 2). The population structure of the strains was visualized by means of a cgMLST-based phylogenetic tree. The isolates grouped according to serotypes and STs (Fig. 1). Except for two STEC O27:H30 (B20-22 recovered from red deer from Slovenia, and C15-2 from red deer from Switzerland, respectively), they were phylogenetically clearly distinct, with ≥ 15 different alleles between each pair of neighboring isolates (Fig. 1).
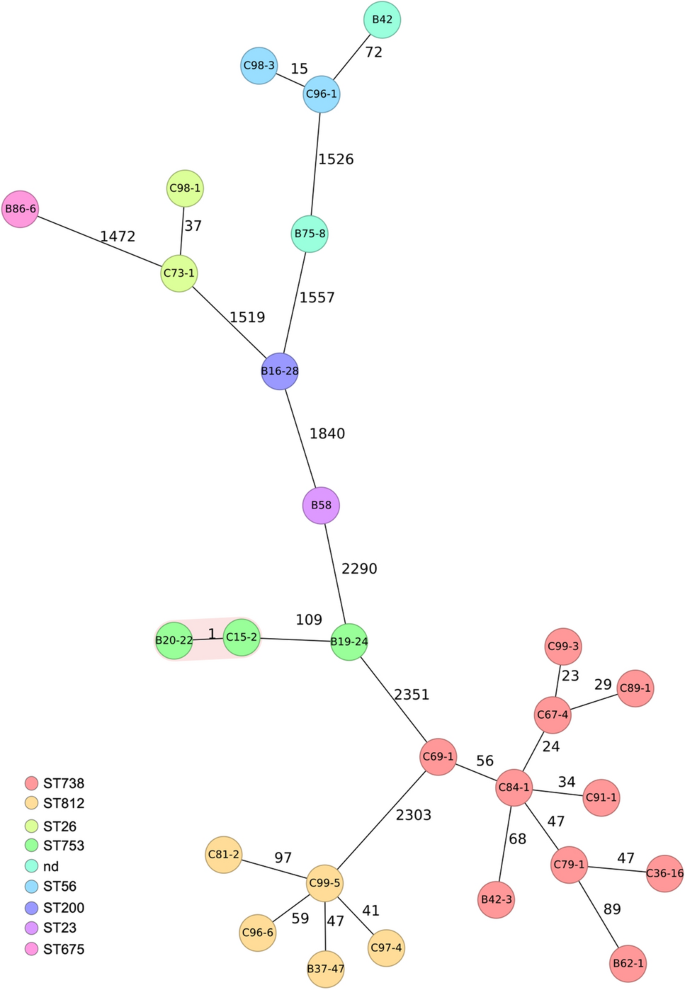
Phylogenetic relationship of 27 Shiga toxin-producing Escherichia coli (STEC) isolated from game meat based on their core genome multilocus sequence type (cgMLST) allelic profiles. The minimum spanning tree was generated using SeqSphere (Ridom GmbH). Numbers on connecting lines indicate the number of allele differences between two strains. The colors of the circles represent STs according to the Warwick scheme (http://enterobase.warwick.ac.uk). Strain IDs are indicated in the circles.
Stx subtypes and additional virulence determinants
Subtyping of the stx genes revealed that five of the 27 isolates (19%) harboured stx1c only (Table 2). Eighteen of 27 (67%) carried stx2 genes only; i. e. stx2a (n = 1), stx2b (n = 15), stx2e (n = 1), and stx2g (n = 1). Four of 27 (15%) harboured the combination of stx1c and stx2b genes (Table 2). The stx2a gene, which is associated with severe disease was identified in the O179:H8 isolate B75-8 recovered from wild boar meat.
Besides stx genes, a large number of additional virulence genes were identified among the strains, including genes encoding toxins astA (n = 18), ehxA (n = 12), eilA (n = 3), subAB1 (n = 1), subAB2 (n = 18), sta1 (n = 1), usp (n = 10), and vat (n = 1), and adhesins (air (n = 2), espI (n = 1), espP (n = 1), focC (n = 1), hra (n = 18), iha (n = 21), lpfA (n = 19), ompT (n = 24), papC (n = 5), pic (n = 5), sfaD (n = 1), and yfcV (n = 5). The adhesin gene saa was found only as a partial sequence in one isolate (B75-8) (data not shown).
Further VFs included those associated with iron acquisition fyuA (n = 7), ireA (n = 20), iroN (n = 1), irp2 (n = 5), and sitA (n = 1), with the ability to survive bactericidal serum activity (iss, n = 19), those associated with acidic tolerance (gad, (n = 17), a gene encoding the capsule polysaccharide export protein (kpsE, n = 5), an outer membrane protein complement resistance gene (traT, n = 26), and the tellurite resistance gene terC (n = 27). Notably, none of the isolates in this study carried the eae gene, which is an adhesin that is present in many STEC21.
Some of the VFs mentioned above, including air, eilA, and pic, are also associated with EAEC22. However, the aggR and the aat genes, which are typical molecular predictors of EAEC9, were not detected.
The presence of sta1, encoding the heat stable enterotoxin typically produced by ETEC23, was detected in the O187:H28 isolate B16-28 from red deer meat (Table 2).
Additionally, many of the VFs found among the isolates are associated with ExPEC including chuA (n = 18), focC (n = 1), fyuA (n = 7), ireA (n = 20), irp2 (n = 5), papC (n = 5), sfaD (n = 1), usp (n = 10), vat (n = 1), and yfcV (n = 5) (Table 2).
Antimicrobial resistance genes
All 27 STEC isolates in this study harboured blaEC genes, which are Ambler class C cephalosporinases derived by mutations from ampC24 (data not shown). Further, all isolates contained genes for the E. coli resistance-nodulation-division (RND) efflux pump AcrAB-TolC which is a major contributor to intrinsic resistance to antibiotics and resistance to bile salts which allows bacterial colonization and adaptation to the intestinal tract25 (data not shown).
Discussion
While recent years have seen an increase in the popularity of game meat, there is a concern that this comes with the risk of exposure to zoonotic pathogens, including STEC. STEC constitute part of the microbiota of the gastrointestinal tract of a variety of wild animals and may contaminate the meat during evisceration and skinning procedures, processing, and packaging26,27.
In this study, the presence of stx1 and stx2 genes was detected in 84% of the enrichment cultures, indicating that the overall contamination of game meat with STEC is high. In 23 of the stx-positive meat samples STEC could be isolated. Therefore, with an overall prevalence of 25%, the level of STEC contamination in the present study was considerably higher than the 5.6% STEC prevalence reported in game meat and game meat products in Spain during 2009–2010 and 2010–201128, the 9.9% prevalence in retail game meat from Germany in 200629, and the 10% prevalence in red deer meat samples from the USA in 201330. However, comparative data are still scarce and differences in the testing methodologies of different studies may lead to variations between the results. Nevertheless, the present study provides evidence that the occurrence of STEC in game meat may currently be underestimated.
With one exception (isolate B75-8 O179:H8 harbouring stx2a), none of the isolates contained the virulence genes stx2a, stx2d, or eae, all of which are significantly associated with severe disease in humans8,31. Further, none of the isolates belonged to the so called "top five" serogroups of human pathogenic STEC O157, O26, O103, O111, and O1455, indicating that overall, the pathogenic potential of STEC in game meat is rather low. Nonetheless, other toxin genes, including astA, ehxA, subAB1 and subAB2 found in 18/27, 12/27, 1/27, and 18/27 of the STEC in this study, are also considered important virulence markers for STEC pathogenesis and are frequently detected among human clinical isolates32,33. Notably, the subtilase cytotoxin subtype SubAB2 is an emerging pathogenic factor that is prevalent among human eae-negative STEC and also typically found among STEC from wildlife and small ruminants33,34,35. Moreover, the majority of the isolates (21 of the 27) harboured iha, which is thought to contribute to pathogenicity of eae-negative STEC by facilitating attachment to intestinal cells36. These findings indicate that the STEC occurring in game meat have the potential to cause disease in humans. Notably, STEC O187:H28 ST200 (isolate ID B16-28 recovered from red deer meat) carrying the rare stx2g subtype co-harboured the sta1 gene, a heat stable enterotoxin typically produced by ETEC. Similar hybrid STEC/ETEC O187:H28 have been described recently from free-ranging red deer in Italy37, from flour samples in Germany and Switzerland38,39, and from a small child with diarrhoea in Sweden40. This highlights the importance of hybrid STEC and shows that game meat might serve as vehicle for possible human STEC/ETEC infections.
Interestingly, 24 out of 27 (89%) isolates harboured one or more virulence factors which are characteristic of ExPEC41,42,43. Although infections with the majority of the STEC in this study are less likely to cause severe gastrointestinal symptoms, STEC/ExPEC should be not underestimated due to the possibility of a systemic infection in combination with gastrointestinal disease44.
The most frequently identified STEC serotype in the present study was O146:H28 (ST738) harbouring stx2b. STEC O146 is among the most common non-O157 serogroups associated with human illness in Europe5, and STEC O146:H28 harbouring stx2b were found in 4% of all human non-O157 STEC infections in Switzerland in 201732. STEC O146:H28 has also been identified in raw dog food45 and hulled wheat and rye flour samples in Switzerland46, indicating its wide distribution throughout various ecological niches. As was seen for the majority of the STEC in this study, STEC O146:H28 carried a range of VF associated with extraintestinal pathogenic disease, but was the only serotype to harbour the uropathogenic-specific protein (usp) gene which has been described in E. coli that are linked to pyelonephritis, prostatitis and bacteraemia47. Other STEC described in this study are not commonly associated with human disease but have been recovered from deer and wild boar meat, for example STEC O8:H9, O21:H21 and O27:H30, and O110:H3148,49. Phylogenetic analysis showed that only two STEC O27:H30 isolated were clonal. Highly similar STEC O27:H30 have been observed frequently in deer meat samples in Spain, suggesting an association between O27:H30 and deer28.
Taken together our data indicate that STEC present in game meat are genetically diverse, and that a subset of STEC may have the potential to cause extraintestinal infections in humans.
Finally, in this study, all the isolates carried chromosomal cephalosporinase genes and genes for RND efflux systems that are of clinical significance because they can confer resistance to third generation cephalosporins, aminoglycosides, and phosphonic acid derivatives, all of which are antimicrobials categorized by the World Health Organization (WHO) as critically important in human medicine50. These genes are ubiquitous in E. coli, however, over-expression of intrinsic AcrAB-TolC multi-drug efflux pump genes may lead to multidrug resistance and the likelihood of treatment failure in the case of a systemic infection with STEC/ExPEC44,51.
Conclusions
This study identified game meat as a source of STEC, including STEC with serotypes, stx subtypes and other virulence traits that are associated with human disease.
Promoting awareness among hunters who handle game in the field, game meat manufacturers, and consumers is important to minimize the risk of exposure.
In addition, consumers and professionals within the food hospitality industry should be advised that products made from raw game meat such as tartare, carpaccio, and cured sausages are associated with a potential risk of infectious disease.
Material and methods
Sampling
An overview of the countries of origins and the suppliers of the game meat samples is given in Table 3. Samples originated from chamois (Rupicapra rupicapra), red deer (Cervus elaphus), roe deer (Capreolus capreolus), and wild boar (Sus scrofa), and were obtained during November 2021.
The game meat processing establishment is located in Slovenia and processes domestic and imported hunted game animals and produces game meat cuts which are distributed in European countries (Table 3). During sample collection, lot numbers of the packed meat were noted to exclude that different samples originated from the same animal.
Screening for stx genes
Each sample (10 g) was enriched at a 1:10 ratio in Enterobacteriaceae enrichment (EE) broth (Becton, Dickinson, Heidelberg, Germany) for 24 h at 37 °C. One loopful of each of the enrichment cultures was cultured on sheep blood agar (Difco™ Columbia Blood Agar Base EH; Becton Dickinson AG, Allschwil, Switzerland) using the streak-plate method. The resulting colonies were suspended in 2 ml 0.85% NaCl. Samples were then screened by real-time PCR for stx1 and stx2 using the Assurance GDS® for Shiga Toxin Genes (Bio Control Systems, Bellevue, WA, USA).
Recovery of STEC
In the event of a stx positive PCR result, one loopful of suspension was streaked onto STEC Chromagar plates (CHROMagar, Paris, FR) and Brolacin agar plates (Bio-Rad, Hercules CA, USA) to get single colonies. The plates were incubated at 37 °C overnight. From each plate, 20–180 individual colonies were picked (mauve colonies on STEC Chromagar plates; yellow colonies on Brolacin Agar plates) and suspended in 0.5 ml 0.85% NaCl. The suspensions were pooled in groups of material from ten colonies and screened for stx1 and stx2 genes by real-time PCR (LightCycler R 2.0 Instrument, Roche Diagnostics Corporation, Indianapolis, IN, USA) using the QuantiFast Multiplex PCR Kit (Qiagen, Hombrechtikon, Switzerland) according to the guidelines of the European Union Reference Laboratory (EURL)52. In the event of a positive PCR result for stx1 or stx2, the pool was taken apart and the ten colonies were tested again individually. From plates yielding more than one stx1 and/or stx2 positive colony, one presumptive STEC isolate was randomly chosen for subsequent characterisation by whole genome sequencing (WGS) analysis. If the screening results indicated colonies with different stx types, the different corresponding colonies were included in the further analysis.
DNA extraction and whole genome sequencing
Isolates were grown on sheep blood agar at 37 °C overnight prior to DNA isolation using the DNA blood and tissue kit (Qiagen, Hombrechtikon, Switzerland). The DNA libraries were prepared using a Nextera DNA Flex Sample Preparation Kit (Illumina, San Diego, CA, USA). Whole genome sequencing was performed on an Illumina MiniSeq Sequencer (Illumina, San Diego, CA, USA). The Illumina-read files passed the standard quality checks using the software package FastQC 0.11.7 (Babraham Bioinformatics, Cambridge, UK) and were assembled using the Spades 3.14.1 based software Shovill 1. 1.053, using default settings. The assembly was filtered, retaining contigs > 500 bp and annotated using the NCBI prokaryotic genome annotation pipeline54. Stx types were determined by an in silico PCR using the perl script "in_silico_pcr" (https://github.com/egonozer/in_silico_pcr) with the option "-m, allow one mismatch" activated and primer sets described in the EURL manual for stx genes detection55. The O- and H-types were identified using SerotypeFinder 2.056. The sequence type (ST) of each strain was determined based on seven housekeeping genes using the tool "MLST"57using PubMLST as database (https://pubmlst.org/)58. The genetic relatedness of the isolates was assessed through core genome MLST (cgMLST) analyses using the Ridom SeqSphereC + software version 5.1.0 (https://www.ridom.de/seqsphere/). A minimum spanning tree (MST) was generated for visualization with the threshold for cluster identification set to ≤ 10 alleles between a pair of isolates, according to the Ridom SeqSphereC+ software. The virulence gene profiles and antimicrobial resistance genes were determined using VirulenceFinder 2.059 and Resistance Gene Identifier (RGI) 4.2.260. Subtilase cytotoxin A and B subunit genes and subtilase cytotoxin subtypes subAB1 and subAB2 were determined using Abricate61 with standard settings and an in-house made database containing nucleotide sequences of subAB1 genes from E. coli 98NK2 (Acc. No. AY258503) and subAB2 genes from E. coli ED32 (Acc. No. JQ994271). Presence of the adhesin gene saa was determined using tblastn with the Saa protein as input62 and the sequenced genomes as query. A cut off of > 70% identity with a 70% alignment rate was applied.
Data availability
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accessions JAPMME000000000 to JAPMNE000000000. The versions described in this paper are versions JAPMME000000000 to JAPMNE000000000. Accession numbers for the individual isolates from this study can be found as Supplementary Table S1 online. The BioProject number is PRJNA903888.
References
Majowicz, S. E. et al. Global incidence of human Shiga toxin-producing Escherichia coli infections and deaths: A systematic review and knowledge synthesis. Foodborne Pathog. Dis. 11, 447–455 (2014).
Karch, H., Tarr, P. I. & Bielaszewska, M. Enterohaemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol. 295, 405–418 (2005).
Bai, X., Scheutz, F., Dahlgren, H. M., Hedenström, I. & Jernberg, C. Characterization of clinical Escherichia coli strains producing a novel Shiga toxin 2 subtype in Sweden and Denmark. Microorganisms. 9, 2374 (2021).
Gill, A. et al. Characterization of atypical Shiga toxin gene sequences and description of Stx2j, a new subtype. J. Clin. Microbiol. 60, e0222921 (2022).
Koutsoumanis, K. et al. Pathogenicity assessment of Shiga toxin-producing Escherichia coli (STEC) and the public health risk posed by contamination of food with STEC. EFSA J. 18, e05967 (2020).
Yang, X. et al. Escherichia coli strains producing a novel Shiga toxin 2 subtype circulate in China. Int. J. Med. Microbiol. 310, 151377 (2020).
Fuller, C. A., Pellino, C. A., Flagler, M. J., Strasser, J. E. & Weiss, A. A. Shiga toxin subtypes display dramatic differences in potency. Infect. Immun. 79, 1329–1337 (2011).
Persson, S., Olsen, K. E. P., Ethelberg, S. & Scheutz, F. Subtyping method for Escherichia coli Shiga toxin (verocytotoxin) 2 variants and correlations to clinical manifestations. J. Clin. Microbiol. 45, 2020–2024 (2007).
Kaper, J. B., Nataro, J. P. & Mobley, H. L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 (2004).
Paton, A. W., Srimanote, P., Talbot, U. M., Wang, H. & Paton, J. C. A new family of potent AB(5) cytotoxins produced by Shiga toxigenic Escherichia coli. J. Exp. Med. 200, 35–46 (2004).
Bielaszewska, M. et al. Characterisation of Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect. Dis. 11, 671–676 (2011).
Mellmann, A. et al. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS ONE 6, e22751 (2011).
Gigliucci, F. et al. Genomic characterization of hlyF-positive Shiga toxin–producing Escherichia coli, Italy and the Netherlands, 2000–2019. Emerg. Infect. Dis. 27, 853 (2021).
Nüesch-Inderbinen, M., Cernela, N., Wüthrich, D., Egli, A. & Stephan, R. Genetic characterization of Shiga toxin producing Escherichia coli belonging to the emerging hybrid pathotype O80:H2 isolated from humans 2010–2017 in Switzerland. Int. J. Med. Microbiol. 308, 534–538 (2018).
Soysal, N. et al. Enterohemorrhagic Escherichia coli hybrid pathotype O80:H2 as a new therapeutic challenge. Emerg. Infect. Dis. 22, 1604–1612 (2016).
Augustin, J.-C. et al. Risk factors for sporadic infections caused by Shiga toxin-producing Escherichia coli: A systematic review and meta-analysis. Microb. Risk Anal. 17, 100117 (2021).
Joseph, A., Cointe, A., MarianiKurkdjian, P., Rafat, C. & Hertig, A. Shiga toxin-associated hemolytic uremic syndrome: A narrative review. Toxins. 12, 67 (2020).
Kintz, E., Brainard, J., Hooper, L. & Hunter, P. Transmission pathways for sporadic Shiga-toxin producing E. coli infections: A systematic review and meta-analysis. Int. J. Hyg. Environ. Health. 220, 57–67 (2017).
Marescotti, M. E., Caputo, V., Demartini, E. & Gaviglio, A. Discovering market segments for hunted wild game meat. Meat Sci. 149, 163–176 (2019).
Gomes-Neves, E., Abrantes, A. C., Vieira-Pinto, M. & Müller, A. Wild game meat—A microbiological safety and hygiene challenge. Curr. Clin. Microbiol. Rpt. 8, 31–39 (2021).
Karmali, M. A. Emerging public health challenges of Shiga toxin-producing Escherichia coli related to changes in the pathogen, the population, and the environment. Clin. Infect. Dis. 64, 371–376 (2017).
Boisen, N. et al. Genomic characterization of enteroaggregative Escherichia coli from children in Mali. J. Infect. Dis. 205, 431–444 (2012).
Crossman, L. C. et al. A commensal gone bad: Complete genome sequence of the prototypical enterotoxigenic Escherichia coli strain H10407. J. Bact. 192, 5822–5831 (2010).
Mammeri, H., Poirel, L., Fortineau, N. & Nordmann, P. Naturally occurring extended-spectrum cephalosporinases in Escherichia coli. Antimicrob. Agents Chemother. 50, 2573–2576 (2006).
Thanassi, D. G., Cheng, L. W. & Nikaido, H. Active efflux of bile salts by Escherichia coli. J. Bact. 179, 2512–2518 (1997).
Soare, C. et al. The microbial condition of Scottish wild deer carcasses collected for human consumption and the hygiene risk factors associated with Escherichia coli and total coliforms contamination. Food Microbiol. 108, 104102 (2022).
Branciari, R., Onofri, A., Cambiotti, F. & Ranucci, D. Effects of animal, climatic, hunting and handling conditions on the hygienic characteristics of hunted roe doer (Caprelous capreolus L.). Foods. 9, E1076 (2020).
Díaz-Sánchez, S. et al. Detection and characterization of Shiga toxin-producing Escherichia coli in game meat and ready-to-eat meat products. Int. J. Food Microbiol. 160, 179–182 (2012).
Martin, A. & Beutin, L. Characteristics of Shiga toxin-producing Escherichia coli from meat and milk products of different origins and association with food producing animals as main contamination sources. Int. J. Food Microbiol. 146, 99–104 (2011).
Magwedere, K. et al. Incidence of Shiga toxin-producing Escherichia coli strains in beef, pork, chicken, deer, boar, bison, and rabbit retail meat. J. Vet. Diagn. Investig. 25, 254–258 (2013).
Byrne, L., Adams, N. & Jenkins, C. Association between Shiga toxin-producing Escherichia coli O157:H7 stx gene subtype and disease severity, England, 2009–2019. Emerg. Infect. Dis. 26, 2394–2400 (2020).
Nüesch-Inderbinen, M. et al. Serotypes and virulence profiles of Shiga toxin-producing Escherichia coli strains isolated during 2017 from human infections in Switzerland. Int. J. Med. Microbiol. 308, 933–939 (2018).
Fierz, L., Cernela, N., Hauser, E., Nüesch-Inderbinen, M. & Stephan, R. Characteristics of Shigatoxin-producing Escherichia coli strains isolated during 2010–2014 from human infections in Switzerland. Front. Microbiol. 8, 1471 (2017).
Michelacci, V. et al. A new pathogenicity island carrying an allelic variant of the subtilase cytotoxin is common among Shiga toxin producing Escherichia coli of human and ovine origin. Clin. Microbiol. Infect. 19, E149–E156 (2013).
Nüesch-Inderbinen, M. T. et al. Prevalence of subtilase cytotoxin-encoding subAB variants among Shiga toxin-producing Escherichia coli strains isolated from wild ruminants and sheep differs from that of cattle and pigs and is predominated by the new allelic variant subAB2-2. Int. J. Med. Microbiol. 305, 124–128 (2015).
Tarr, P. I. et al. Iha: A novel Escherichia coli O157: H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect. Immun. 68, 1400–1407 (2000).
Lauzi, S. et al. Free-ranging red deer (Cervus elaphus) as carriers of potentially zoonotic Shiga toxin-producing Escherichia coli. Transbound. Emerg. Dis. 69, 1902–1911 (2022).
Boss, R. & Hummerjohann, J. Whole genome sequencing characterization of Shiga toxin-producing Escherichia coli isolated from flour from Swiss retail markets. J. Food Prot. 82, 1398–1404 (2019).