Adverse Events From COVID-19 Vaccines Much Higher Than All ... - The Epoch Times
The COVID-19 pandemic and the rapid rollout of COVID-19 vaccines—including two with novel mRNA technology—raised concerns about the safety of these new vaccines.
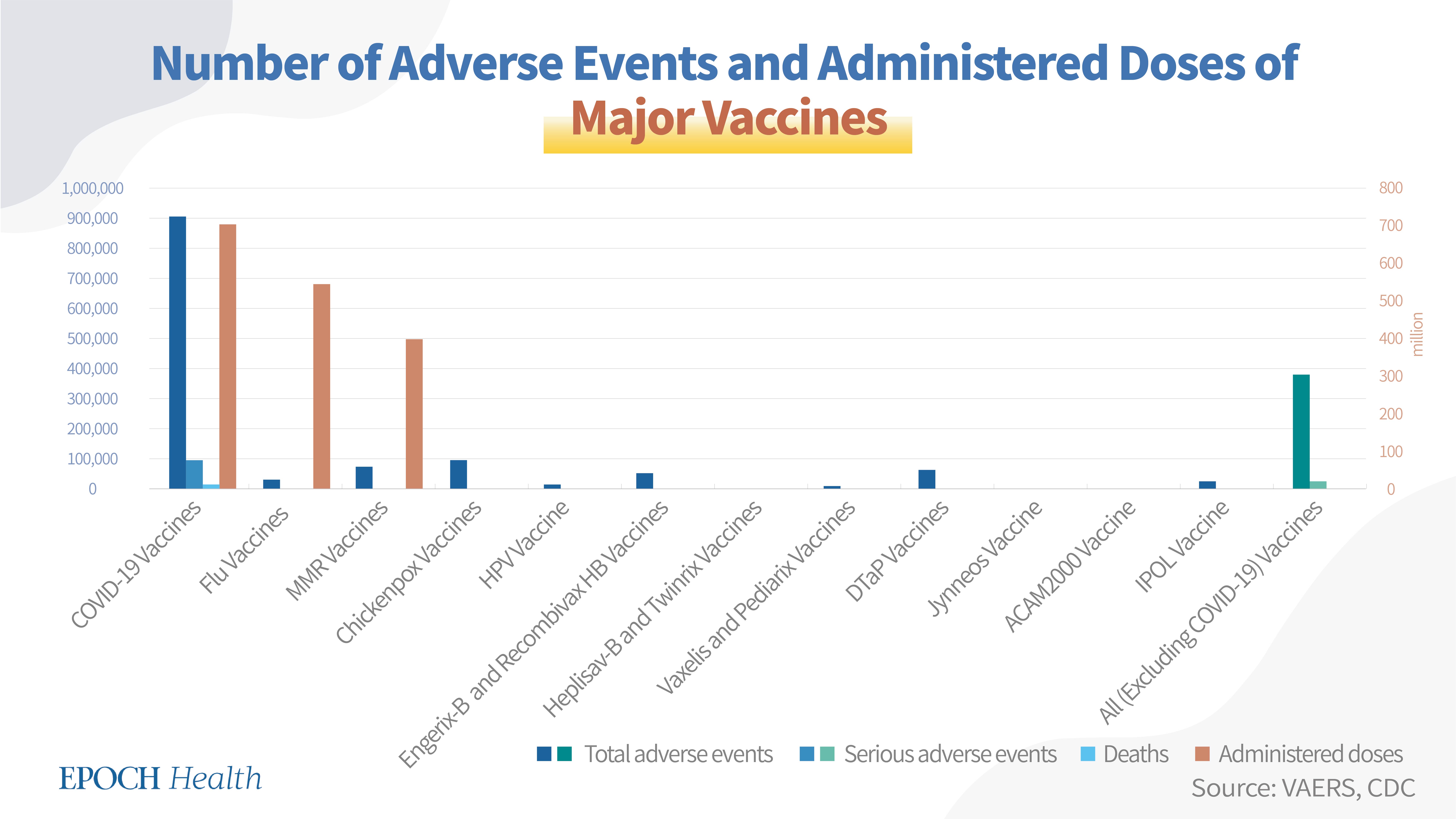
The CDC's Vaccine Adverse Event Reporting System (VAERS) is a postmarketing vaccine surveillance program, to which anyone can report vaccine adverse events. A comparison between the adverse event reports of COVID-19 vaccines and those of other major vaccines such as those for seasonal flu, hepatitis B, HPV, measles, and polio, shows more adverse events reported in the VAERS system for the COVID-19 vaccines compared to the others in terms of the number of administered doses.
For the purpose of this article, the numbers of total VAERS adverse events, serious adverse events, and deaths (considered serious adverse events) of the major vaccines are examined and compared. In places where a particular vaccine's total number of administered doses is unavailable, the vaccine coverage ratio is used to offer a general idea about the vaccine's estimated doses.
I. COVID-19 Vaccine Adverse Events Reported in VAERS
Currently, four COVID-19 vaccines are used mainly for the primary series, and two additional bivalent vaccines are used as boosters. Among the vaccines used for the primary series, there are two mRNA COVID-19 vaccines: the monovalent Pfizer-BioNTech and Moderna COVID-19 vaccines. Both vaccines' respective bivalent formulations have also been authorized by the U.S. Food and Drug Administration (FDA) as boosters. The Janssen COVID-19 vaccine, manufactured by Johnson & Johnson, is a viral vector vaccine, and the recently authorized Novavax COVID-19 vaccine is protein-based with an adjuvant.
VAERS adverse event report numbers:
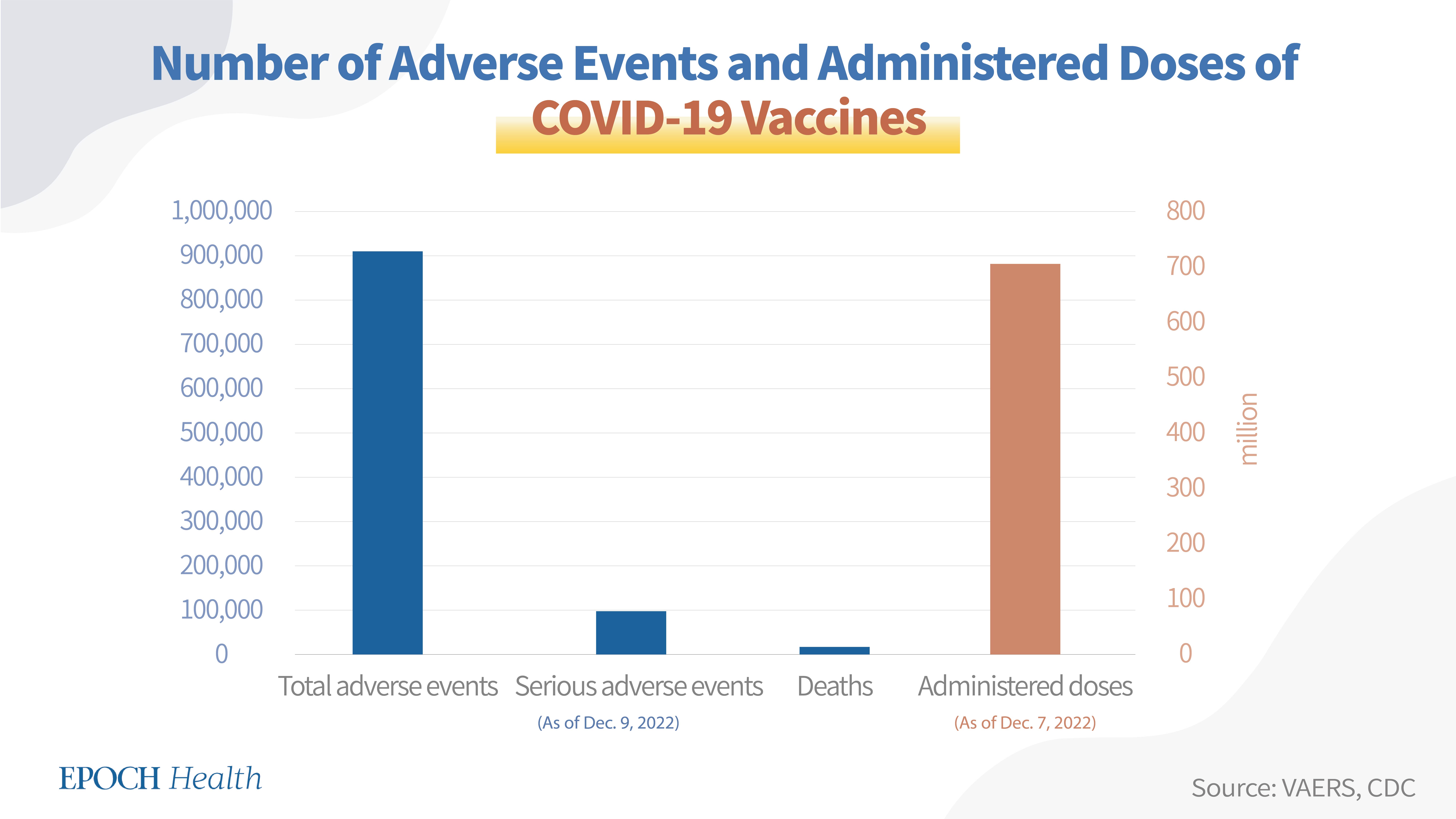
As of Dec. 9, 2022, a total of 909,868 adverse events had been reported regarding all six COVID-19 vaccines in the U.S., including 96,140 serious adverse events and 15,733 deaths.
Vaccine administration and dosage numbers:
The monovalent Pfizer-BioNTech and Moderna COVID-19 vaccines and the Novavax COVID-19 vaccine are currently approved for two doses for the primary series, whereas the Janssen COVID-19 vaccine only requires one dose.
Both bivalent Pfizer-BioNTech and Moderna COVID-19 vaccines are recommended as booster doses.
According to the CDC, as of Dec. 7, 2022, the total number of administered doses of all six COVID-19 vaccines was 703,984,126.
II. Seasonal Influenza Vaccines:
Among the four types of flu viruses, namely A, B, C, and D, influenza viruses A and B are the main cause of the seasonal flu.
There are nine types of flu vaccines available for the 2022-23 season such as FluLaval Quadrivalent and Fluzone Quadrivalent. All nine are quadrivalent vaccines, meant to protect against four different flu viruses, including two flu A viruses and two flu B viruses.
As the flu viruses are constantly changing, the CDC makes annual predictions about the next prevailing flu virus strains for the vaccine manufacturers to develop and produce the vaccines.
VAERS adverse event report numbers:
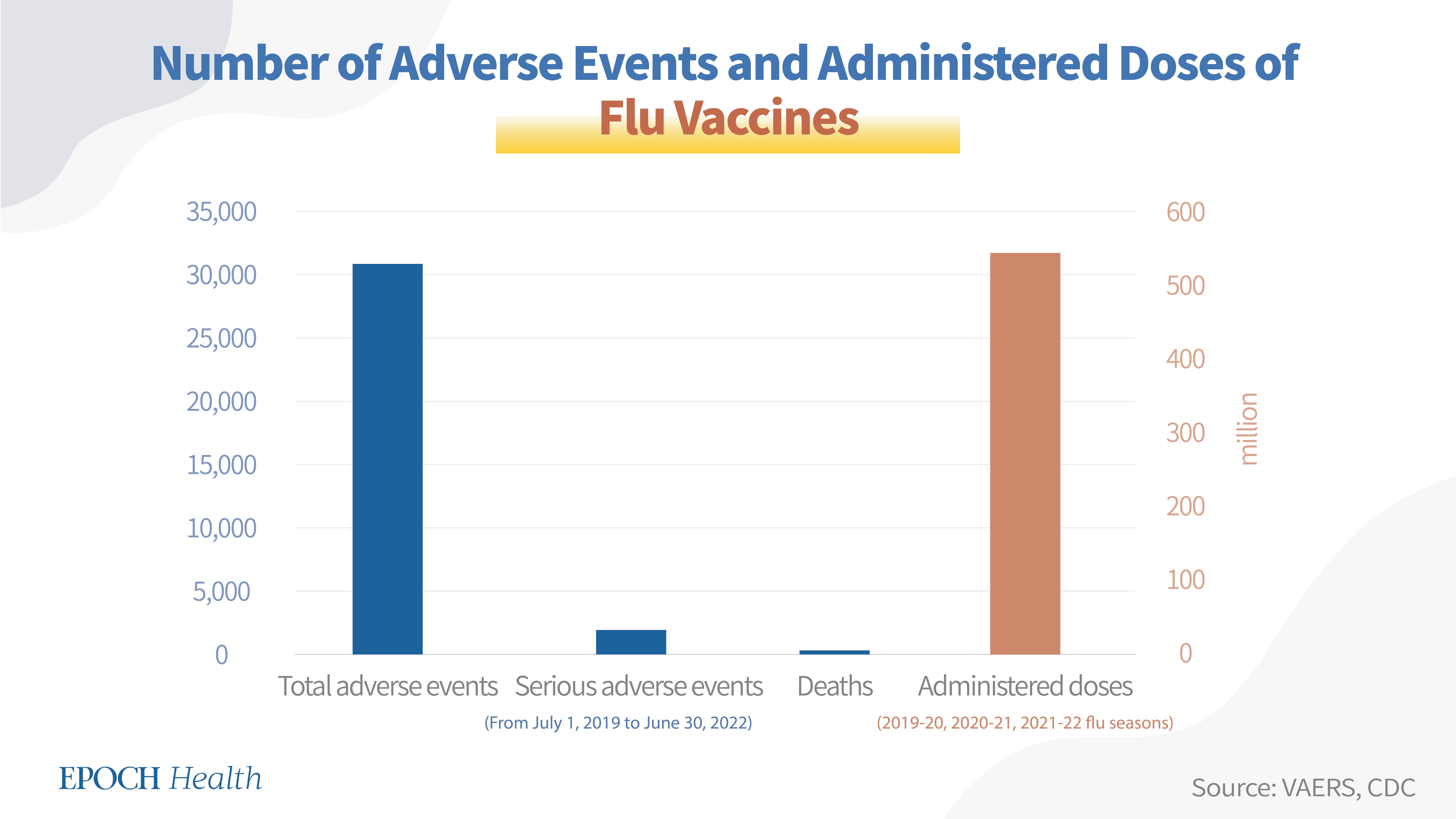
Since seasonal flu vaccines are different from season to season, they are treated as one group for the purpose of calculating VAERS adverse event numbers. According to VAERS, from July 1, 2019, to June 30, 2022 (three flu seasons) a total of 30,848 adverse events had been reported regarding the seasonal flu vaccines, including 1,906 serious adverse events and 235 deaths.
Vaccine administration and dosage numbers:
Children 6 months to 8 years of age are recommended to receive two doses of flu vaccines in one flu season, whereas people older than 8 years of age need only one dose per flu season.
According to the CDC, the seasonal flu vaccine doses distributed during the 2019-20, 2020-21, and 2021-22 seasons were 175.2 million, 194.4 million, and 175.6 million, respectively. Therefore, a total of 545.2 million doses of seasonal flu vaccines were administered in the U.S. for these three seasons.
III. Vaccines for Measles, Mumps, and Rubella
Measles is a highly contagious infectious respiratory disease caused by the measles virus. Its common symptoms include a full-body rash, fever, malaise, cough, and conjunctivitis.
Mumps is a contagious viral disease caused by the mumps virus. Typically affecting the glands on each side of the face, its symptoms may include fever, headache, malaise, and muscle pain.
Rubella—also known as German measles—is a viral infection with a characteristic red rash. It's usually mild, with a low fever and sore throat.
Currently, two FDA-approved measles-mumps-rubella (MMR) vaccines are available for use in the U.S., including M-M-R II and PRIORIX. They are fully interchangeable, to prevent measles, mumps, and rubella.
1. M-M-R II (Measles, Mumps, and Rubella Vaccine)
The combined measles-mumps-rubella (M-M-R II) vaccine, manufactured by Merck & Co., Inc., has been in use in the U.S. for four decades. Prior to that, Merck was producing three separate monovalent vaccines for these three diseases.
VAERS adverse event report numbers
According to VAERS, as of Dec. 9, 2022, a total of 72,681 adverse events had been reported regarding the M-M-R II vaccine, including 5,141 serious adverse events and 243 deaths.
Vaccine administration and dosage number
The currently approved dosage for the M-M-R II vaccine is two doses. Over 265 million doses of the vaccine have been distributed in the U.S., over the past 40 years.
2. PRIORIX (Measles, Mumps, and Rubella Vaccine, Live)
Marketed under the trade name PRIORIX, (measles, mumps, rubella vaccine (live), and manufactured by GlaxoSmithKline Biologicals SA., the FDA approved the vaccine on June 3, 2022, to be used in individuals one year of age and older.
VAERS adverse event report numbers
According to VAERS, as of Dec. 9, 2022, a total of 115 adverse events had been reported regarding the PRIORIX vaccine, including 14 serious adverse events but no deaths.
Vaccine administration and dosage number
The currently approved dosage for the PRIORIX vaccine is two doses. As the vaccine was approved only six months ago, there's no public information on the number of its administered doses. However, according to the CDC, nearly 10 million doses of MMR vaccines have been administered in the U.S. every year.
VAERS reports on both MMR vaccines
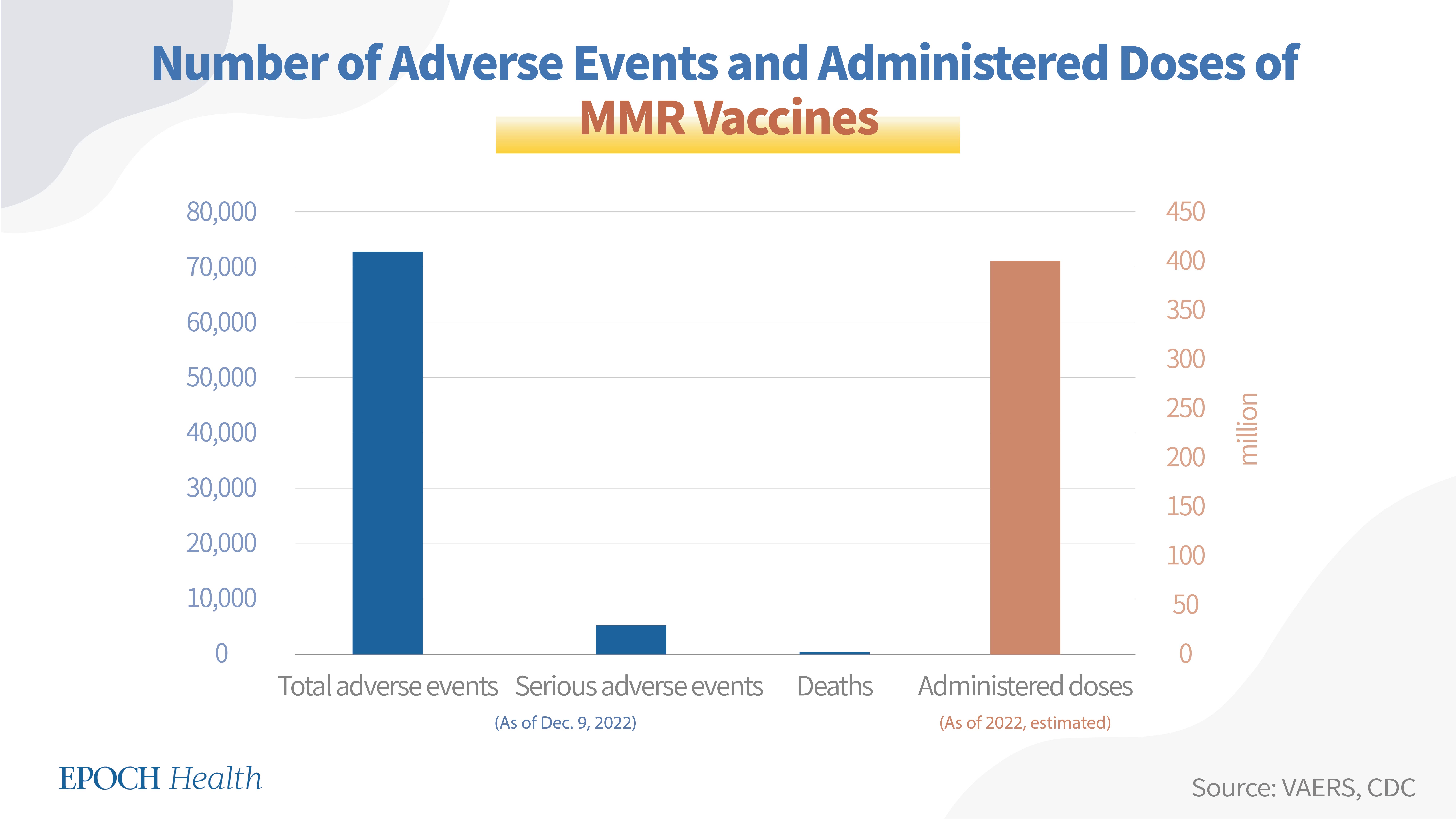
Overall, a total of 72,796 adverse events had been reported regarding both approved MMR vaccines in the U.S., including 5,155 serious adverse events and 243 deaths (see table below).
Since nearly 10 million doses of MMR vaccines have been administered in the U.S. every year, and the M-M-R II® vaccine has been in use for 40 years, the estimated MMR vaccine doses administered for these four decades are nearly 400 million.
IV. Chickenpox Vaccines
Varicella (chickenpox) is a highly contagious infection caused by the varicella-zoster virus (VZV). Its main symptom is an itchy, blister-like rash, which initially appears on the chest, back, and face, before spreading over the entire body.
Currently, there are two FDA-approved vaccines for use to prevent chickenpox in the U.S.—ProQuad, and VARIVAX.
1. ProQuad (Measles, Mumps, Rubella, and Varicella Virus Vaccine, Live)
Marketed under the trade name ProQuad, the measles, mumps, rubella, and varicella (MMRV) vaccine manufactured by Merck was approved by the FDA in 2005 to be used in children 1 to 12 years of age.
As its name implies, in addition to chickenpox, the MMRV vaccine also protects against measles, mumps, and rubella.
The currently approved dosage for the ProQuad vaccine is two doses.
VAERS adverse event report numbers
According to VAERS, as of Dec. 9, 2022, a total of 18,151 adverse events had been reported regarding the ProQuad vaccine, including 488 serious adverse events and 22 deaths.
2. Varivax (Varicella Virus Vaccine, Live)
Unlike the ProQuad vaccine, the Varicella Virus Vaccine Live (Varivax) only prevents chickenpox. It's manufactured by Merck Shark & Dohme Corp. and was approved by the FDA on Mar. 17, 1995, for use in people 12 months of age or older.
The currently approved dosage for the Varivax vaccine is two doses.
VAERS adverse event report numbers
According to VAERS, as of Dec. 9, 2022, a total of 79,067 adverse events had been reported regarding the Varivax vaccine, including 3,175 serious adverse events and 160 deaths.
VAERS reports on both chickenpox vaccines
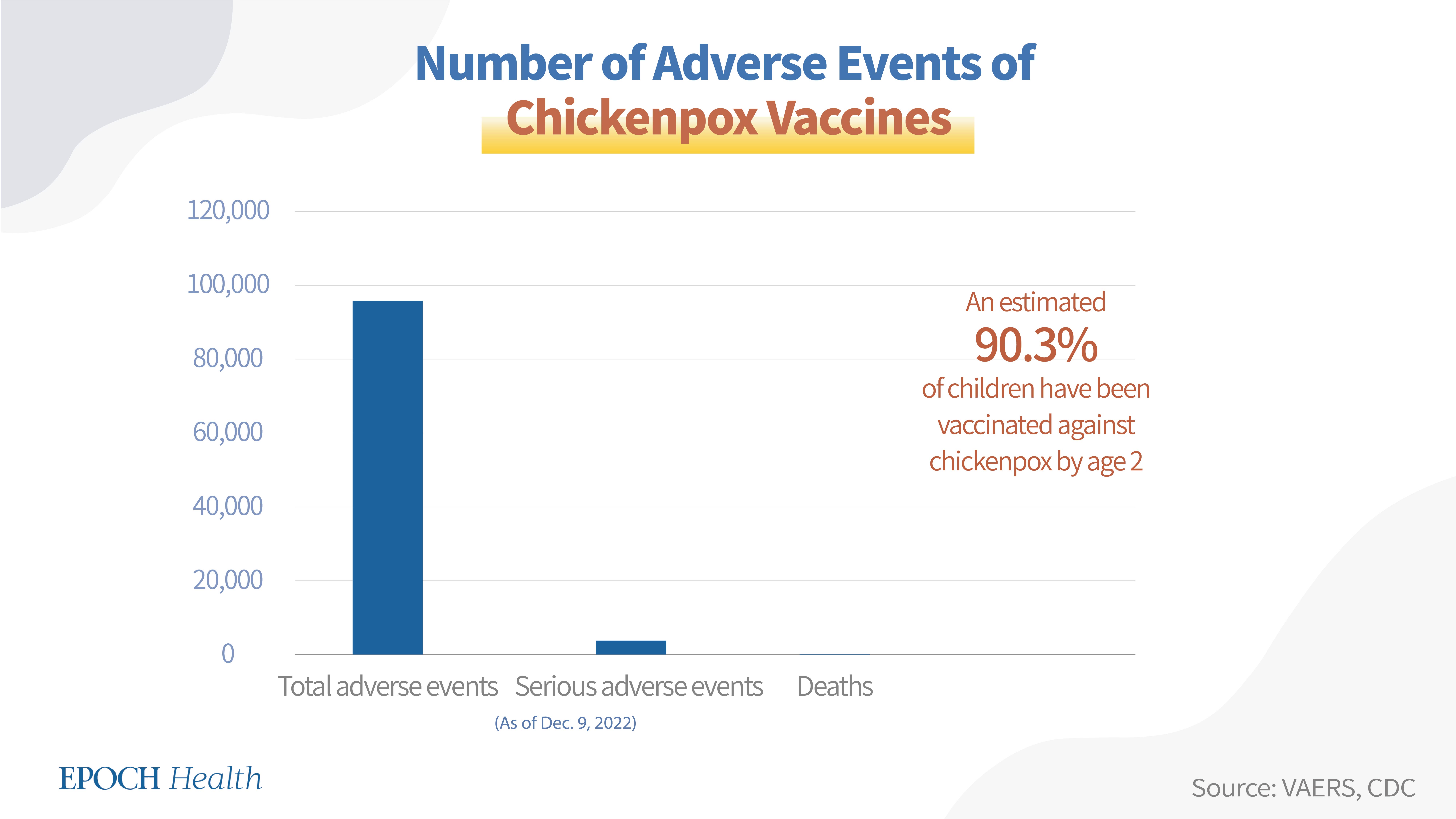
Overall, a total of 95,677 adverse events had been reported regarding both approved chickenpox vaccines in the U.S., including 3,649 serious adverse events and 182 deaths.
Chickenpox vaccine coverage in the U.S.
An estimated 90.3 percent of children have been vaccinated (i.e., with at least one dose) against chickenpox by age 24 months, according to the CDC.
V. Human Papillomavirus (HPV) Vaccine
There are over 200 types of human papillomavirus (HPV)—most of them causing warts (skin or mucous membrane growths)—however, some types can cause cervical and other cancers. HPV can spread between people through skin-to-skin contact.
There is currently one FDA-approved HPV vaccine in distribution in the U.S. Marketed under the brand name Gardasil-9, this 9-valent HPV vaccine is manufactured by Merck for use in individuals aged 9 to 45 years to prevent nine types of HPV. However, the CDC doesn't recommend vaccinating people over the age of 27 for HPV.
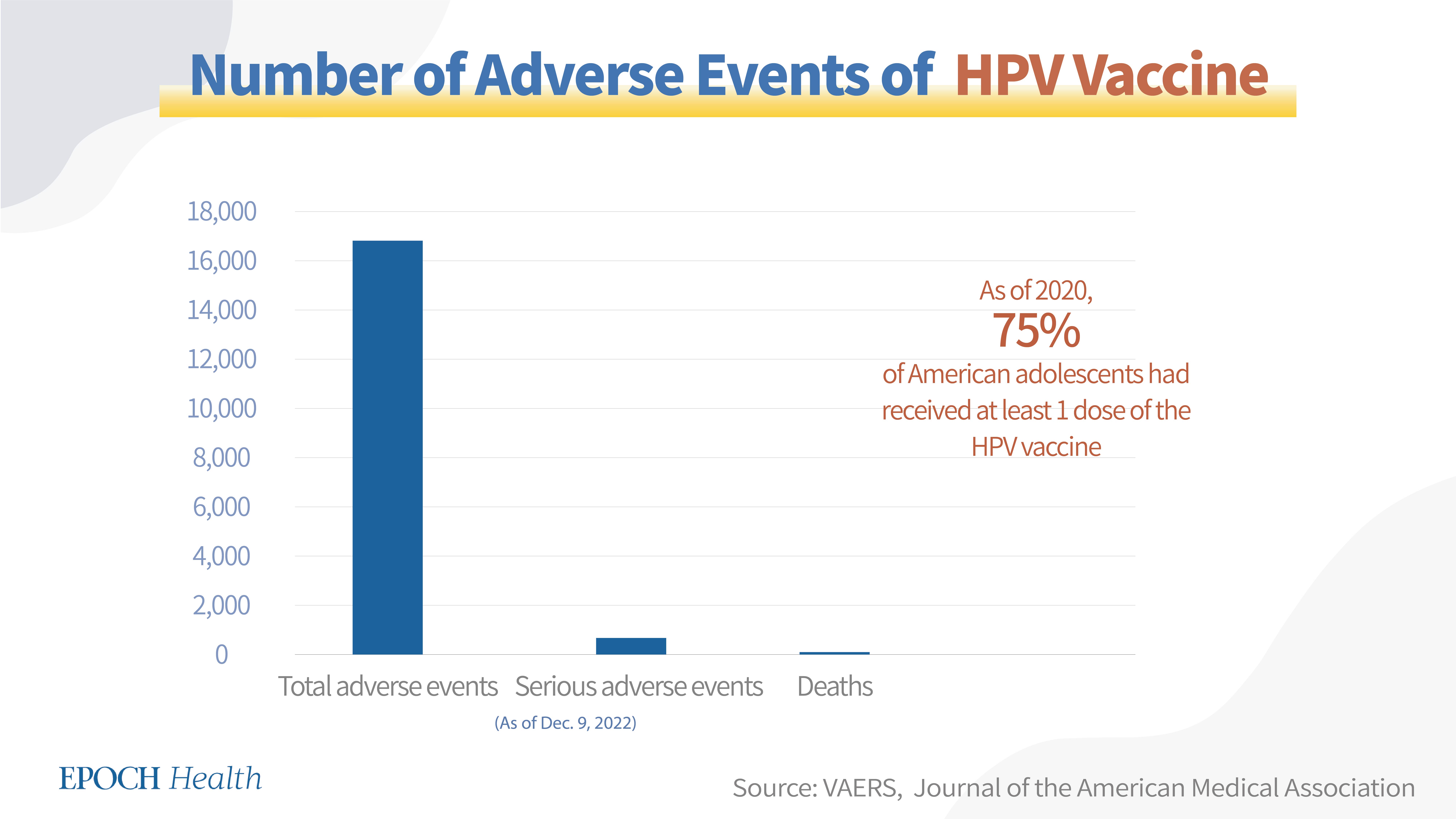
VAERS adverse event report numbers
Gardasil-9 has been the only HPV vaccine in use since 2016. According to VAERS, as of Dec. 9, 2022, a total of 16,805 adverse events had been reported regarding this vaccine, including 630 serious adverse events and 30 deaths (see chart below).
HPV vaccine coverage and dosage in the U.S.
The CDC recommends two doses of the Gardasil-9 vaccine for people that receive the first dose before turning 15 years of age and three doses for people who get the first dose after 15 years of age.
As of 2020, 75 percent of American adolescents had received at least 1 dose of the HPV vaccine.
VI. Hepatitis B Vaccines
Hepatitis B is a severe liver disease caused by the hepatitis B virus (HBV). There are two types of hepatitis B infections—acute and chronic. In the case of chronic hepatitis B infection, the HBV remains in a patient's body for a long period of time. Chronic hepatitis B patients may not experience any symptoms, but the illness can eventually lead to liver cancer or death.
In the U.S., there are five FDA-approved hepatitis B vaccines for adults and two exclusively for infants.
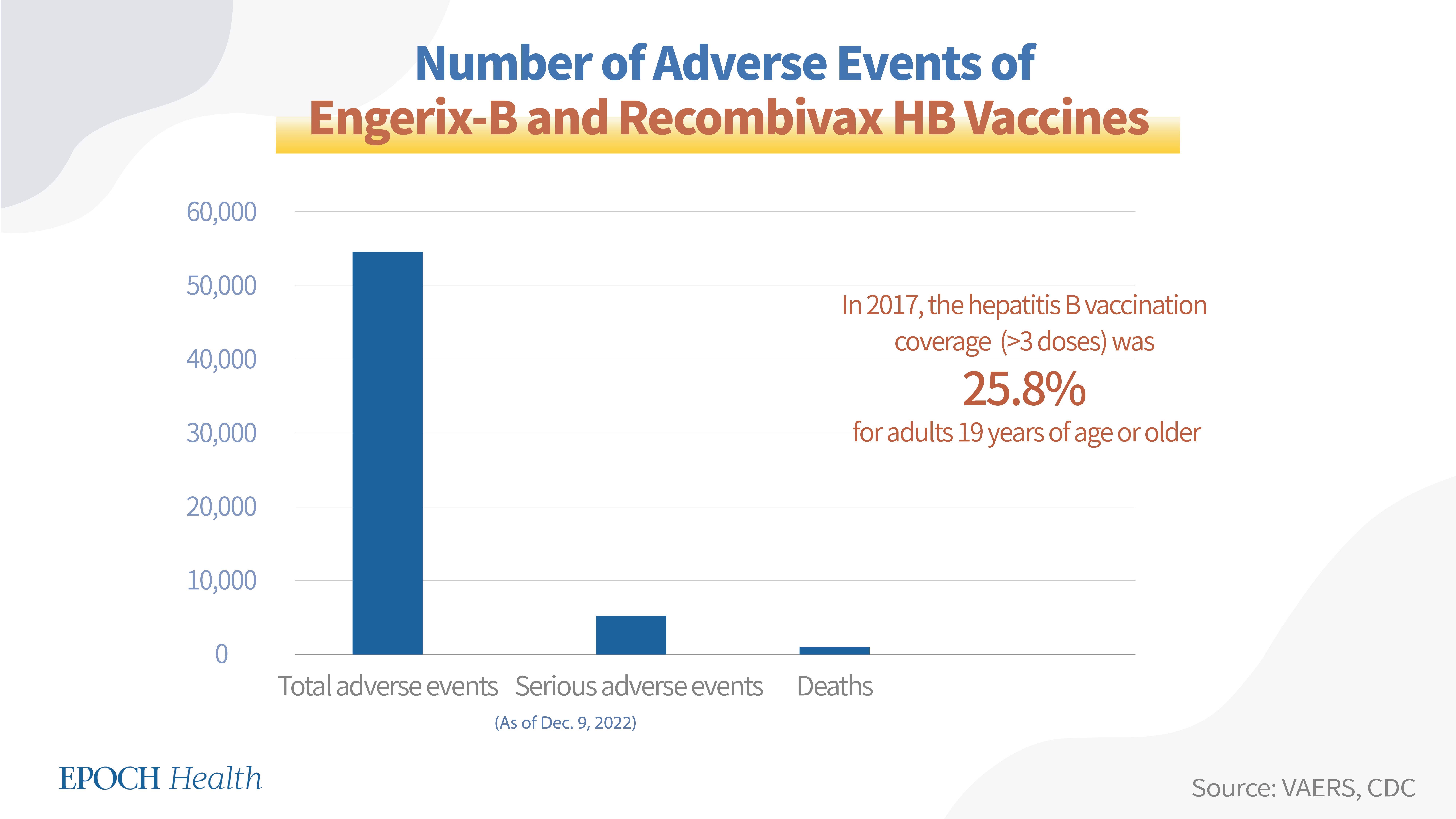
1. Engerix-B and Recombivax HB (for infants, children, and adults)
Hepatitis B vaccines used for infants, children (>1 year), and adults include Engerix-B and Recombivax HB. The recombinant vaccine Engerix-B has been approved by the FDA to be used to prevent infection caused by all known subtypes of HBV. Engerix-B, Recombivax HB is also a recombinant vaccine that can be used to prevent infection caused by all known subtypes of HBV.
According to VAERS, as of Dec. 9, 2022, a total of 54,549 adverse events had been reported regarding the Engerix-B and Recombivax HB vaccines, including 5,159 serious adverse events and 803 deaths (see chart below).
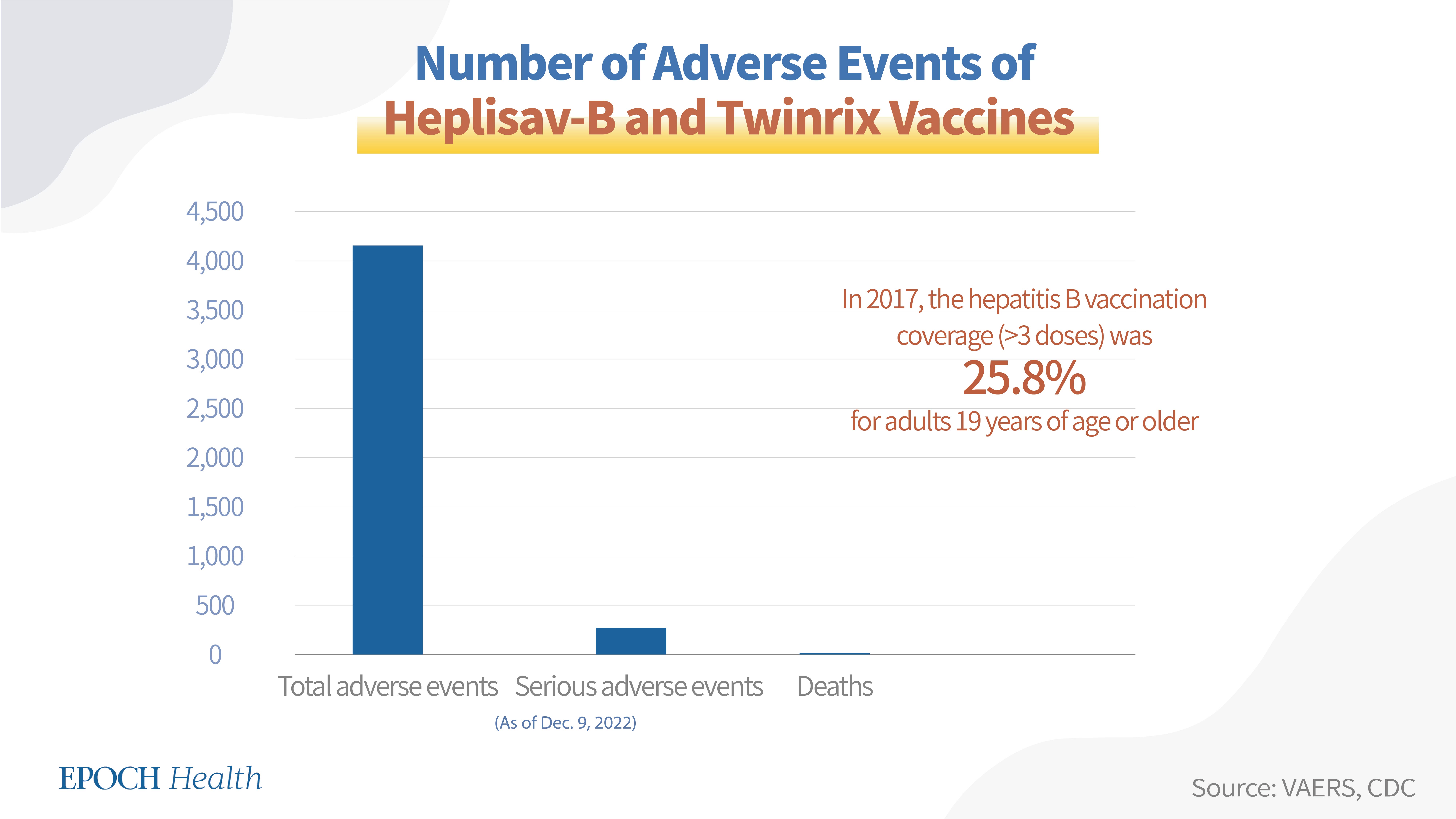
2. Heplisav-B, Pre-Hevbrio, and Twinrix (for adults)
Heplisav-B, Pre-Hevbrio, and Twinrix (for Adults) are the hepatitis B vaccines used in individuals 18 years of age and older. Heplisav-B is an adjuvanted recombinant vaccine used to prevent infection caused by all known subtypes of HBV. Pre-Hevbrio is a recombinant vaccine for the prevention of all known subtypes of HBV, approved by the FDA in November 2021, but thus far, its information hasn't been entered into the VAERS system. Twinrix is a hepatitis A and hepatitis B combination vaccine.
According to VAERS (see chart below), as of Dec. 9, 2022, a total of 4,158 adverse events had been reported regarding the Heplisav-B and Twinrix vaccines, including 269 serious adverse events and 10 deaths.
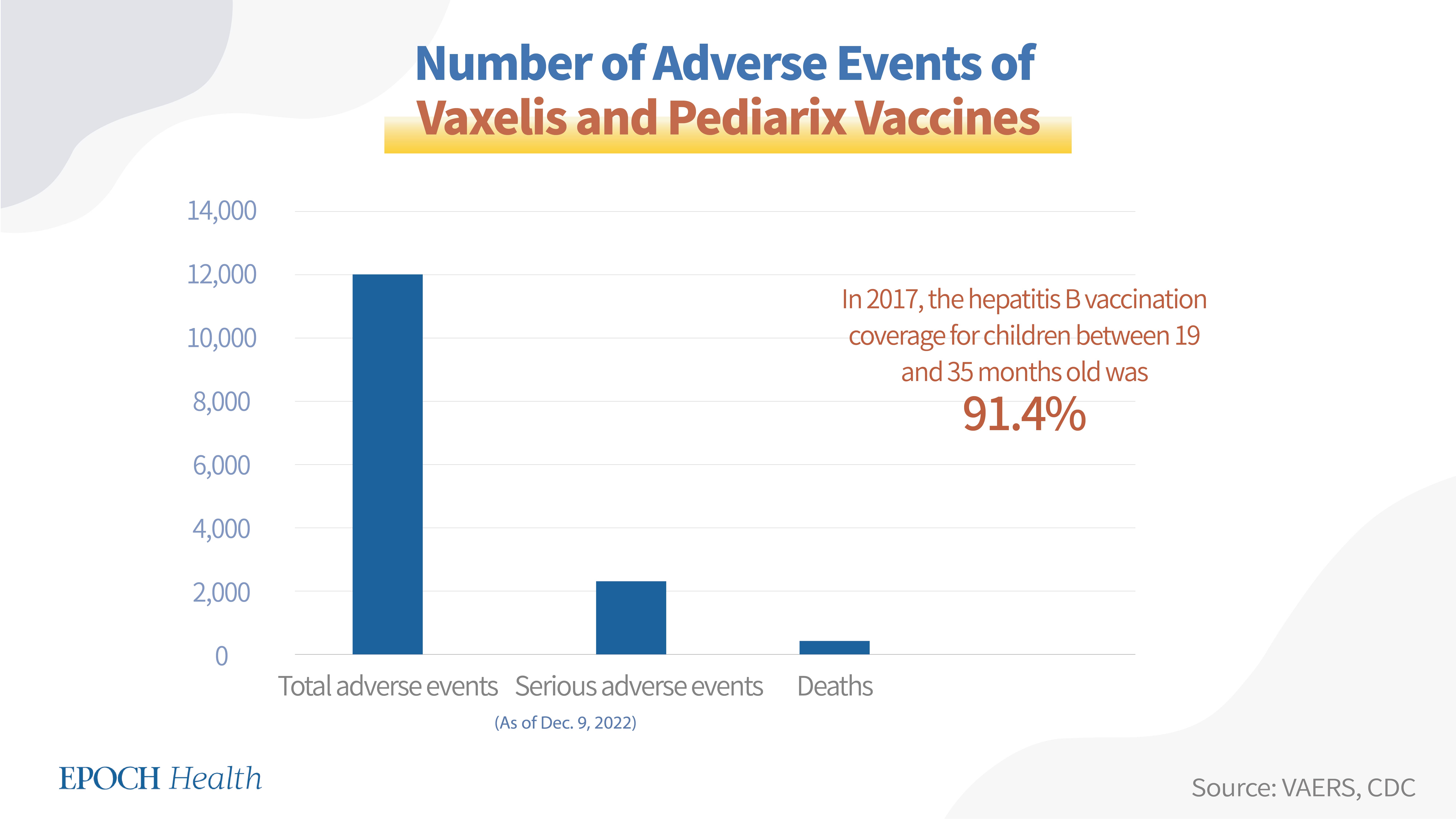
3. Vaxelis and Pediarix (for infants and children)
There are two vaccines that protect infants and children against multiple illnesses, including hepatitis B. Vaxelis protects against diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and Haemophilus influenzae type B (Hib) infection in children 6 weeks through 4 years of age. Pediarix protects against diphtheria, tetanus, pertussis, hepatitis B, and poliomyelitis in children 6 weeks through 6 years of age.
According to VAERS, as of Dec. 9, 2022, a total of 12,021 adverse events had been reported regarding both Vaxelis and Pediarix, including 2,313 serious adverse events and 419 deaths.
Hepatitis B vaccine coverage and dosage in the U.S.:
Hepatitis B vaccines are given in 2, 3, or 4 doses. Among all hepatitis B vaccines, the 2-dose vaccine brand is Heplisav-B and the 3-dose vaccine brands are Pre-Hevbrio, Twinrix, Engerix-B, Recombivax HB, Vaxelis, and Pediarix. According to the CDC, in 2017, the hepatitis B vaccination coverage (>3 doses) was 25.8 percent for adults 19 years of age or older, and its coverage for children between 19 and 35 months old was 91.4 percent.
VII. DTaP (Diphtheria, Tetanus, Pertussis) Vaccines
DTaP vaccines are used to prevent diphtheria, tetanus, and pertussis. Diphtheria is a serious infectious disease caused by the bacterium Corynebacterium diphtheriae. Tetanus (aka lockjaw) is a bacterial infection caused by Clostridium tetani. Pertussis (aka whooping cough and 100-day cough) is a highly contagious infectious disease caused by Bordetella pertussis.
In the U.S., DTaP vaccines are only for children, as other vaccines that prevent diphtheria, tetanus, and pertussis are used in older children, adolescents, and adults. There are currently seven pediatric DTaP vaccines licensed and used in the U.S., including Daptacel, Infanrix, Kinrix, Pentacel, Quadracel, Vaxelis, and Pediarix. Some of these can protect against other infections as well.
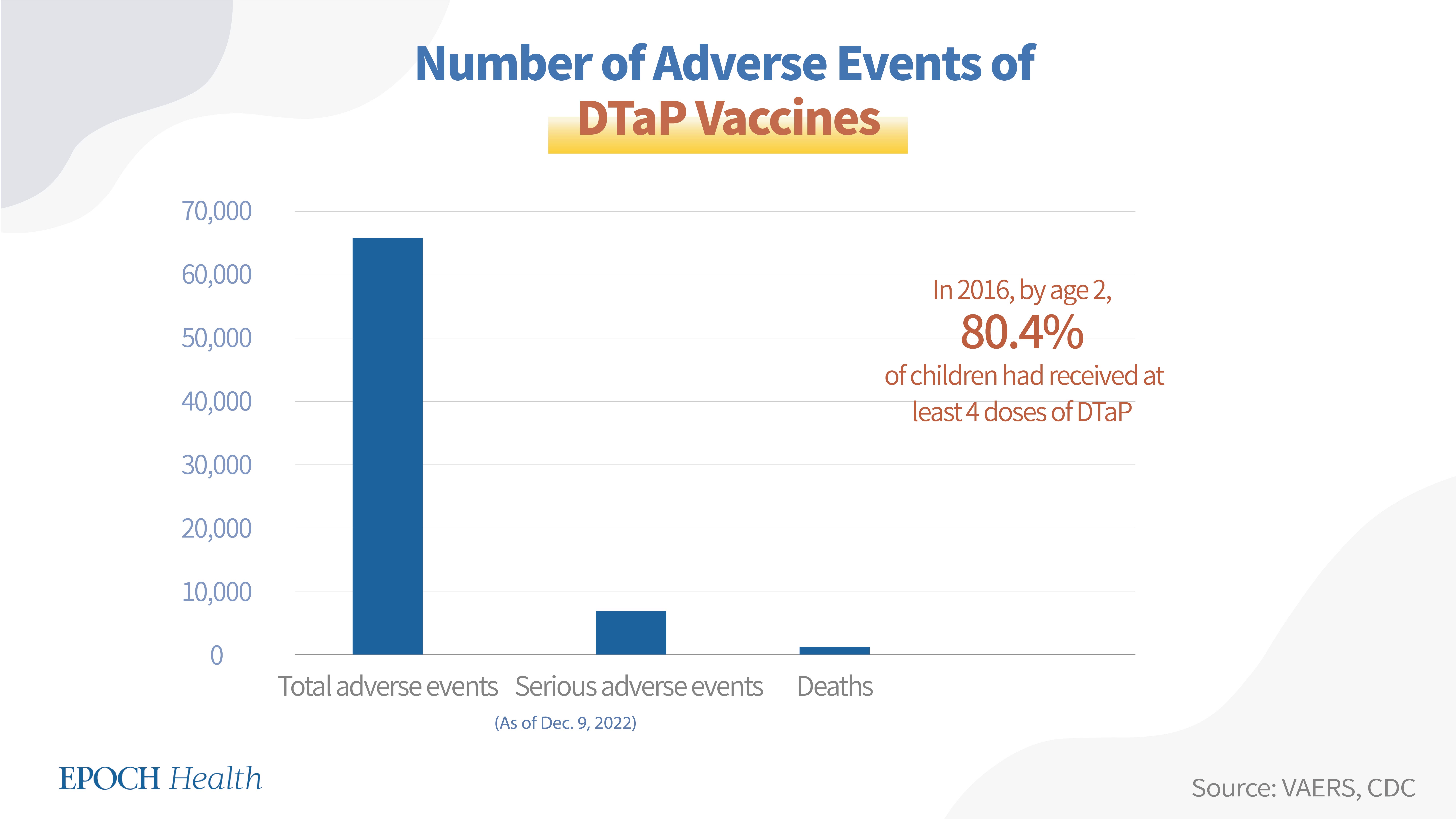
VAERS adverse event report numbers
According to VAERS, as of Dec. 9, 2022, a total of 65,936 adverse events had been reported regarding all seven DTaP vaccines, including 6,831 serious adverse events and 1,016 deaths.
DTaP vaccine coverage and dosage in the U.S.
American children receive five doses of a DTaP vaccine from the age of 2 months to 6 years. According to the CDC, in 2016, by age 2, 80.4 percent of children had received at least 4 doses of diphtheria, tetanus, and pertussis vaccines.
VIII. Smallpox and Mpox (Monkeypox) Vaccines
Smallpox is a serious and highly contagious infectious disease caused by the deadly variola virus. Humans had been plagued by this fatal disease for thousands of years, before the invention of smallpox vaccines. After smallpox was eradicated in the U.S., routine vaccination against smallpox stopped in 1972.
However, smallpox vaccines are still available in the U.S., as people at high risk for smallpox infection (e.g., military personnel) are still recommended by the CDC to receive these vaccines. Smallpox vaccines can be used to protect people from mpox (formerly known as monkeypox). The vaccination can provide protection for around 3 to 5 years, after which the vaccine's protection decreases, and people need to get re-vaccinated.
The only two licensed smallpox vaccines in the U.S. include ACAM2000 and Jynneos, which also protects against monkeypox.
1. Jynneos (smallpox and monkeypox vaccine, live, non-replicating)
Jynneos is used for the prevention of smallpox and monkeypox infections in people 18 years and older. It is one of the three smallpox vaccines in the U.S. Strategic National Stockpile (SNS), approved by the FDA in 2019. On Aug. 9, 2022, due to a monkeypox outbreak, the FDA issued an Emergency Use Authorization (EUA) to authorize the use of Jynneos in minors younger than 18 years old.
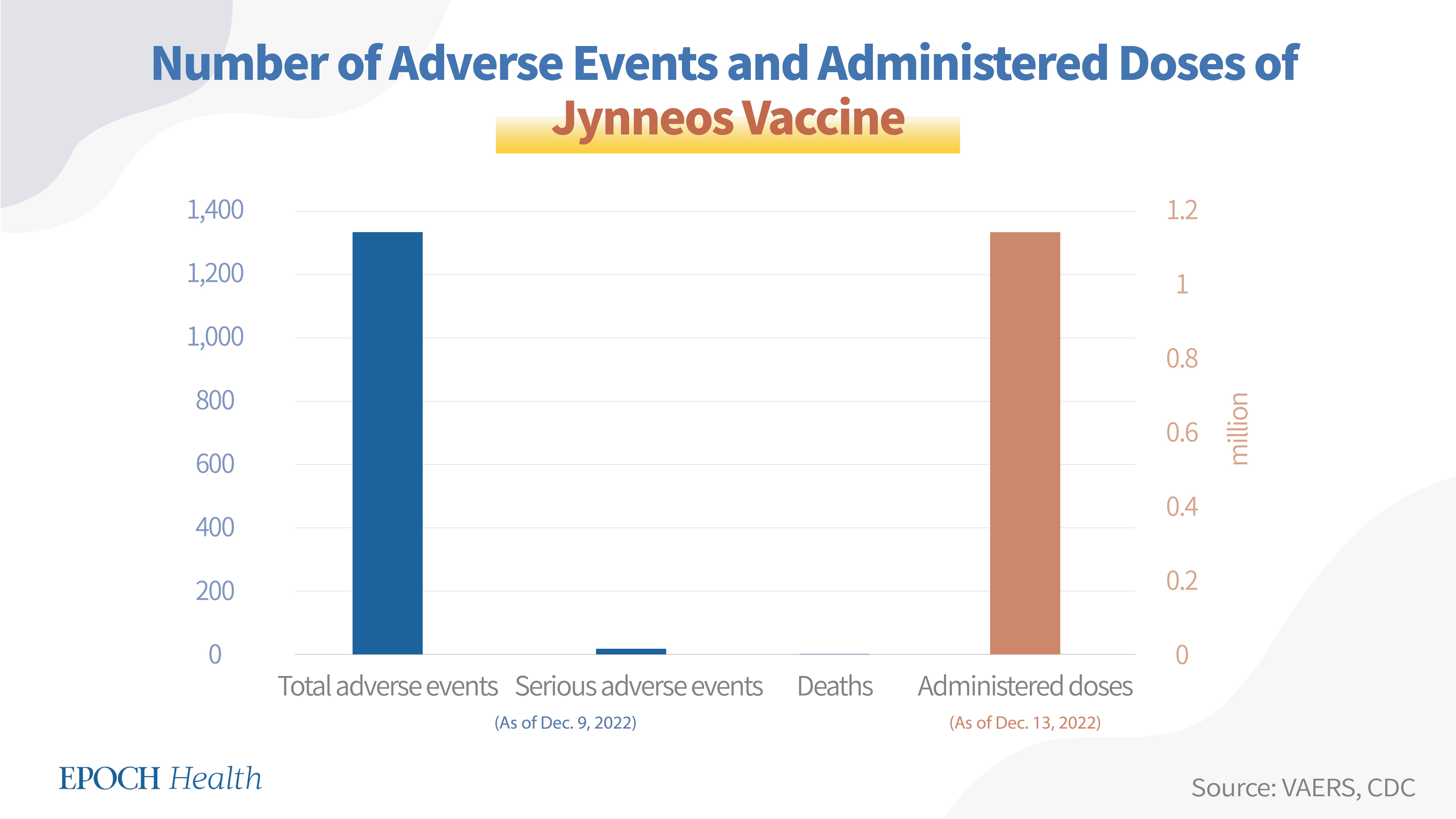
VAERS adverse event report numbers
According to VAERS, as of Dec. 9, 2022, a total of 1,332 adverse events had been reported regarding the Jynneos vaccine, including 15 serious adverse events and 2 deaths.
Vaccine administration and dosage number
The Jynneos vaccine's currently approved dosage is 2 doses. As of Dec. 13, 2022, according to the CDC, a total of 1,141,592 doses of the Jynneos vaccine had been administered in the U.S.
2. ACAM2000 (smallpox vaccinia vaccine, live)
Manufactured by Emergent Product Development Gaithersburg, Inc., ACAM2000 is a second-generation smallpox vaccine in the SNS. The vaccine is made from a live virus called vaccinia, which is related to smallpox but can cause only mild illness. It was approved by the FDA in August 2007.
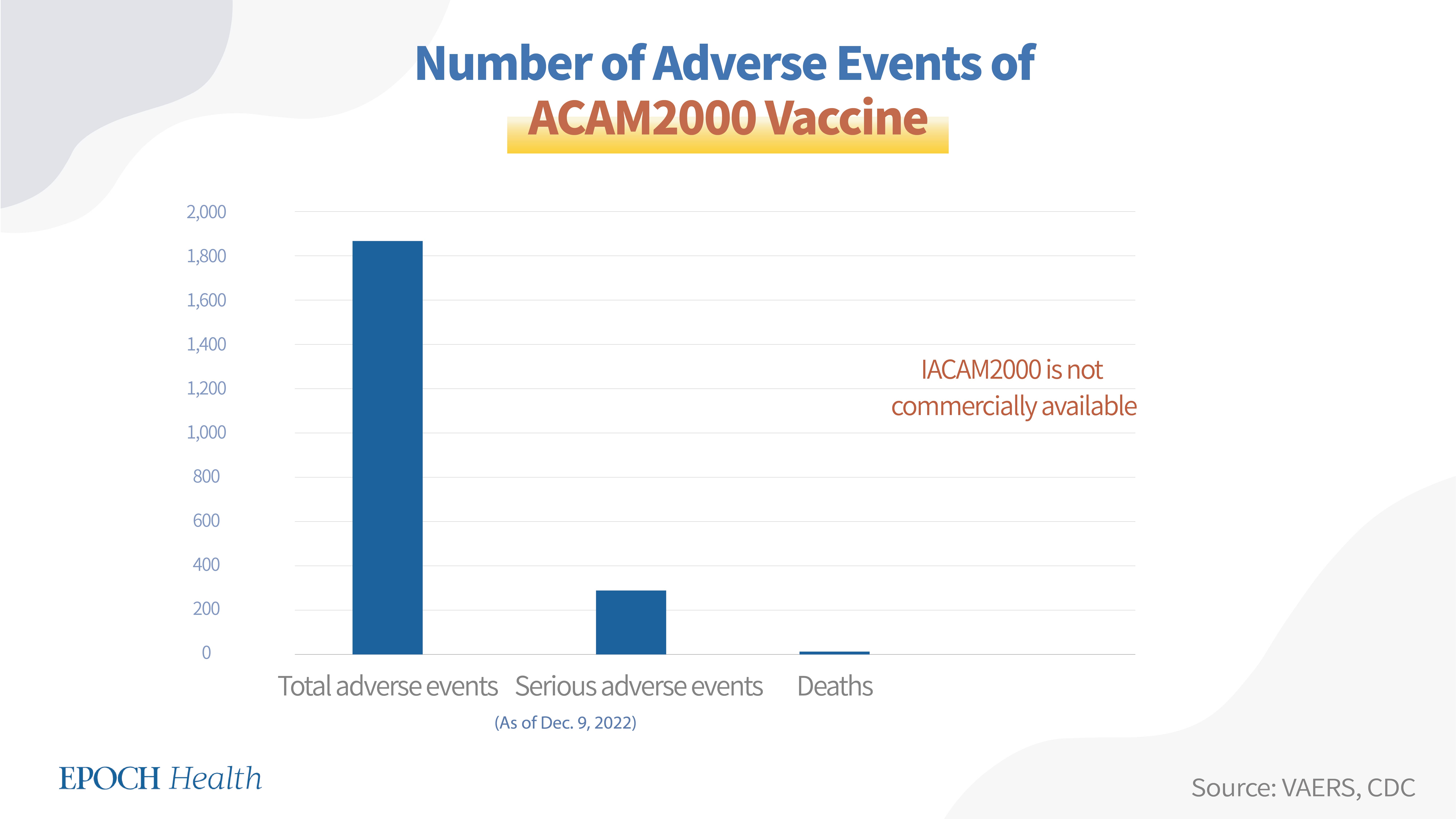
VAERS adverse event report numbers
According to VAERS, as of Dec. 9, 2022, a total of 1,868 adverse events had been reported regarding the ACAM2000 vaccine, including 284 serious adverse events and three deaths.
Vaccine administration situation
The currently approved dosage for ACAM2000 is one dose. As Emergent BioSolutions produces the ACAM2000 vaccine for the SNS, the vaccine is not commercially available. As of 2010, there were over 200 million doses of the ACAM2000 vaccine in the SNS.
VI. Polio Vaccine
Poliomyelitis (polio) is an infection caused by the poliovirus, which spreads from person to person. Polio can be a life-threatening disease causing paralysis.
Poliovirus Vaccine Inactivated (IPOL) is the only polio vaccine currently in use for infants (6 weeks or older), children, and adults in the U.S.
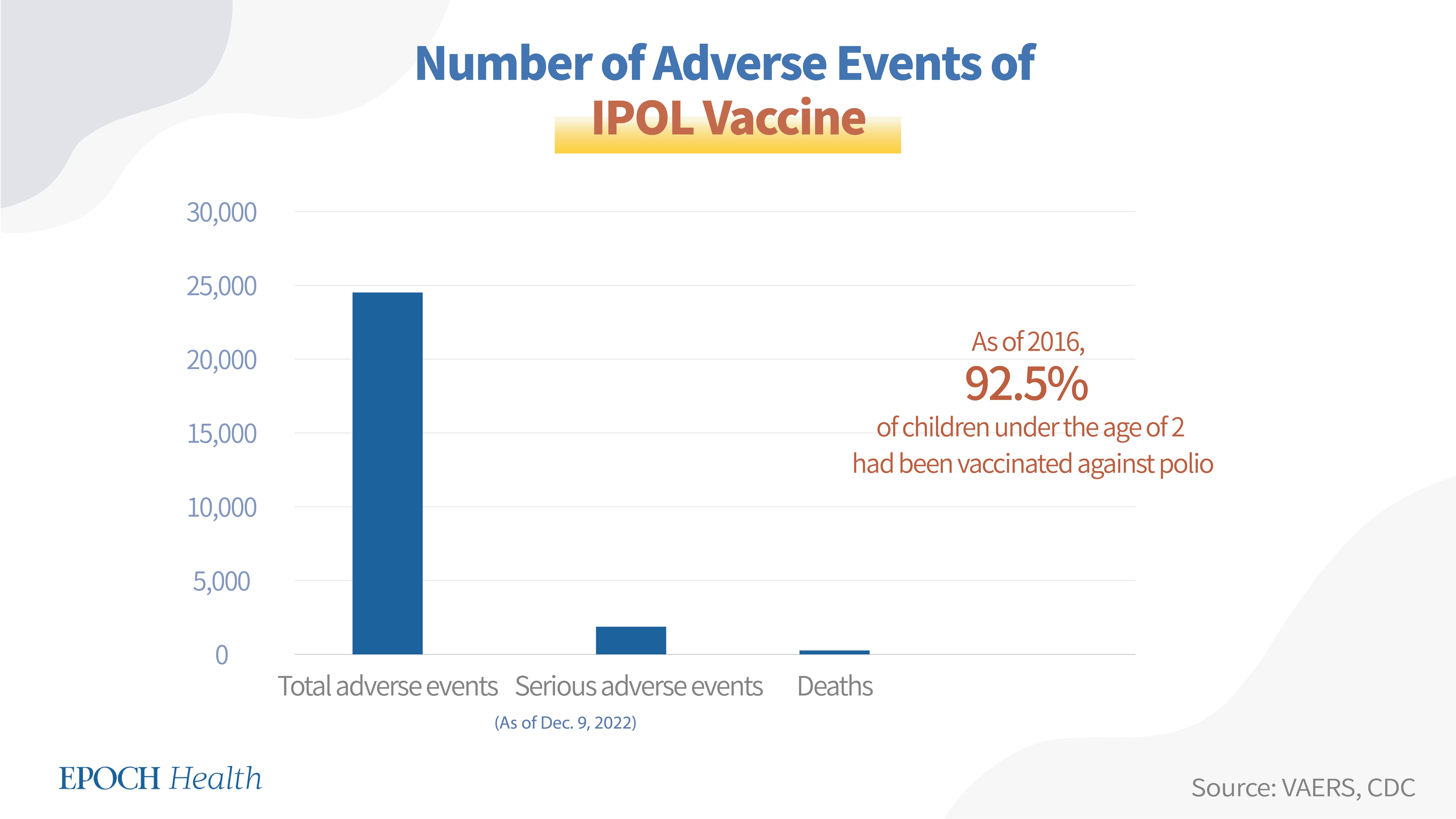
VAERS adverse event report numbers
According to VAERS, as of Dec. 9, 2022, a total of 24,500 adverse events had been reported regarding this vaccine, including 1,847 serious adverse events and 242 deaths.
Polio vaccine coverage and dosage in the United States:
The CDC recommends four doses of the IPOL vaccine for children. As of 2016, 92.5 percent of children under the age of 2 had been vaccinated against polio (by at least three doses).
COVID-19 Vaccines Incur More Adverse Events Than All Other Major Vaccines Combined
According to the VAERS system, the number of adverse events of the COVID-19 vaccines is larger than the total combined number of adverse events of many other major vaccines. As of Dec. 9, 2022, there were 909,868 adverse events related to the COVID-19 vaccines, whereas the total number of adverse events of all the other vaccines examined in this article was 380,490.
*(Please note that the number of adverse events of seasonal flu vaccines used in the calculation is the cumulative number of adverse events over three flu seasons spanning from July 2019 to June 2022. Since seasonal flu vaccines are changing from year to year, only three flu seasons have been selected for presentation for the purpose of this article).
The COVID-19 vaccines had 96,140 cases of serious adverse events compared to 28,058 for the combined other major vaccines, and 15,733 reported deaths related to the COVID-19 vaccines, compared to 3,185 reported deaths related to the combined other major vaccines.


Comments
Post a Comment