Vaccine Development and Surveillance | Bill & Melinda Gates Foundation - Gates Foundation
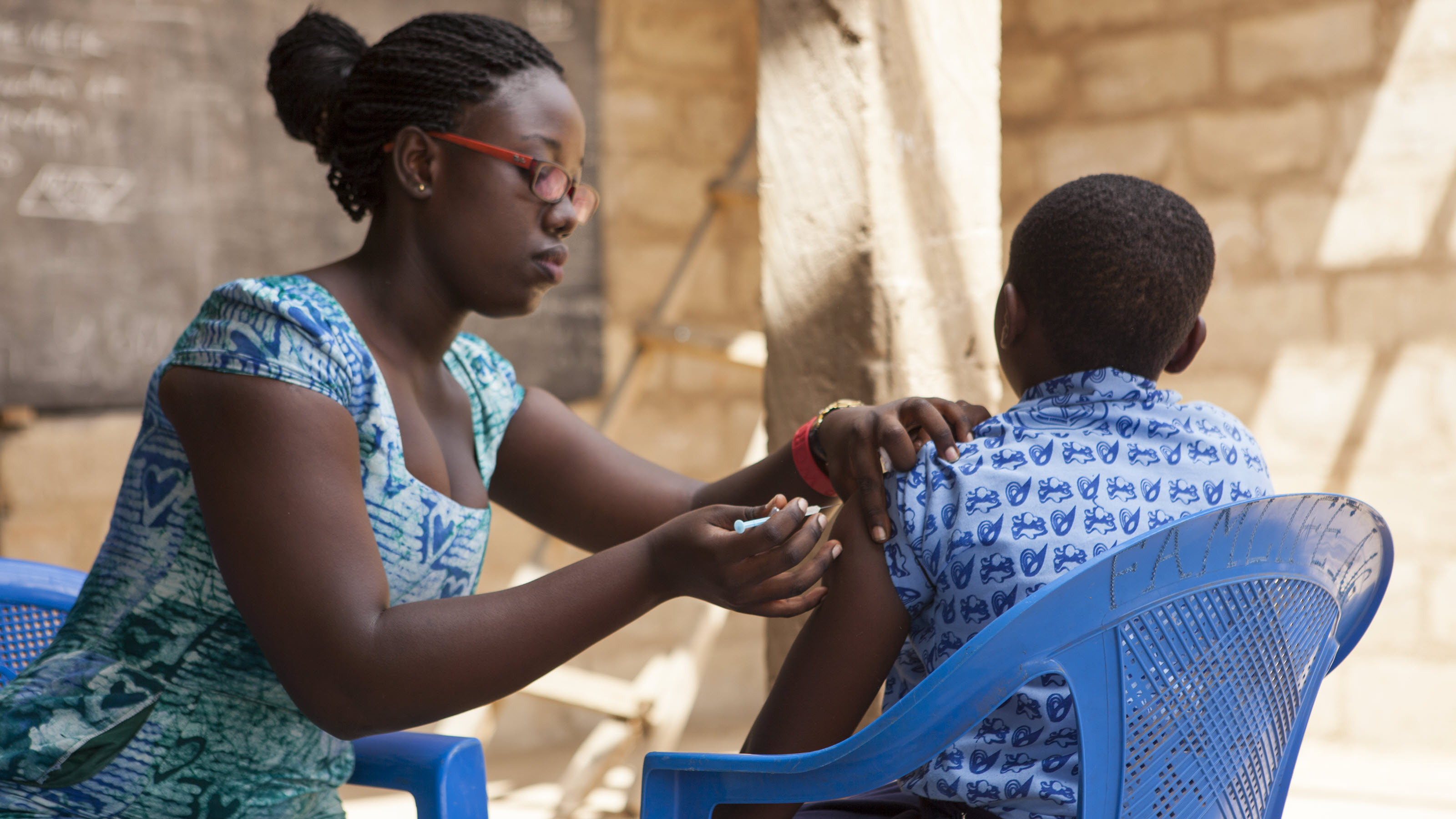
In global health, the focus put on fighting individual diseases has had enormous impact, yet many of the most stubborn challenges we continue to face are shared across disease areas. Whether it's accelerating the development of new vaccines, forecasting the global health challenges of tomorrow, or preparing for epidemics, we must work beyond the scope of one disease area and create durable public goods whose benefits permeate global health.
Vaccines are among the most powerful tools used in combating diseases. Yet despite substantial scientific advances and investment, bringing vaccines to market affordably and reliably remains a challenge. Promising candidates can fail late in development, and existing vaccines can face supply shortages, resulting in wasted time, wasted investments, and missed opportunities to improve human health. The diseases of low-resource settings—whether they are entrenched, like malaria and HIV, or the next outbreak pathogen—are often some of the hardest to address scientifically. They are also often the least attractive commercially. These challenges mean vaccine development for low-resource settings will only be successful if we use innovation in technologies, platforms, processes, and business models to accelerate timelines and reduce costs.
Because developing new vaccines is a lengthy and expensive undertaking, it is particularly important that we understand how to prioritize our efforts. Some diseases lend themselves to vaccine intervention. Others, like the neglected tropical diseases, are best tackled through better deployment of existing interventions. And still others, like noncommunicable diseases, require non-vaccine approaches. Unfortunately, because the quality of our primary data is so poor, it is difficult to determine, for example, how many deaths a malaria vaccine could prevent. Or, in the case of parents who experience the tragedy of losing a child, they may never know the true cause of death. The mystery behind these individual tragedies accumulates into a collective public health conundrum, making it impossible for product developers, governments, and funders to effectively prioritize resources for global and public health.
We believe we can accelerate the impact of vaccines in low-resource contexts by cultivating deep expertise in the vaccine manufacturing process, quality control, and clinical evaluation. This expertise allows us to advise on more effective vaccine development programs and identify new areas of innovation to benefit multiple disease programs.
In addition to technical expertise, we also need to know which problems are the most important to tackle. Trustworthy primary data and high-quality modeling and forecasting allow us to better prioritize the collective resources of global health. By improving the methods for primary data collection, we can give parents the peace of knowing what caused their child to pass away. We can also enhance the aggregated data that policymakers, health workers, funders, and product developers use to make decisions about innovation and implementation.
Finally, we know we need to address the time it takes to develop and deliver vaccines for epidemics. The urgency of epidemics requires solutions in months or even weeks, whereas traditional vaccine development can take years. Addressing this challenge will take innovation. We need to reimagine the way we use our immune systems to combat disease, allowing us to develop both "just-in-time" vaccines for unknown epidemics as well as a store of "just-in-case" vaccines for the next outbreak.

Comments
Post a Comment