Does Vaccination Increase the Risk of Autism Spectrum Disorder? - Cureus
The incidence of autism spectrum disorder (ASD) has risen substantially. This rise has sparked widespread public concern regarding the causes and prevention of the condition. The prevalence of ASD among children aged six to 11 years was 3 per 10,000 in 1991-1992 which increased to 52 per 10,000 in 2001-2002 [1].
Understandably, parents of children with the condition are often angry, feeling guilt, searching for causes, and asking themselves, "Why has this happened?" Many parents blamed themselves, claiming that the problem may be due to dangerous behavior during pregnancy, advanced age at conception, or a genetic element. A narrative that blames an external aspect, on the contrary, appears to be more comfortable; vaccines were the ideal target for their rage and frustration [2].
Wakefield et al. [3] published a report in 1998 describing 12 cases of widespread developmental slowdown linked to gastrointestinal (GI) system symptoms and developmental delay, a fair amount of which occurred shortly after the administration of measles, mumps, and rubella (MMR) vaccine. The theory presented in this case series was that a new variety of ASD was developing and linked to the MMR vaccine; this raised concerns among parents regarding the MMR vaccine's safety and vaccination in general [4,5]. Moreover, a few studies have correlated the number of vaccines added to the children's immunization schedule with the prevalence of ASD diagnosis. Mercury toxicity and modification in immune system function have been the subject of numerous investigations [6]. Following the article by Wakefield et al. on the MMR vaccination-autism link, there has been an upsurge in the antivaccine attitude and vaccine hesitancy in the United States. A lack of readiness to embrace immunization, as shown with pertussis immunization in many countries in the 1970s and 1980s and MMR immunization in the United Kingdom and the United States, resulted in the re-emergence of vaccine-preventable diseases. In the aftermath, acceptability improved [5]. The ideas in the report continue to raise anxiety and challenge vaccine acceptance among parents [6,7].
This systemic review aims to determine any relationship between vaccination and ASD development. We will review multiple articles on vaccination/MMR/ASD and understand their correlation.
Methodology
We conducted our systematic review utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [8].
Database
We started our research on November 15, 2021. We used PubMed and Google Scholar as databases for our data collection.
Search strategy
We included studies on immunization/vaccination and autism/ASD. Our search mechanism included keywords and Medical Subject Headings (MeSH). Table 1 displays the results of each search. The following keywords were used in the literature search: Autism OR autistic disorder OR echolalia OR scripting OR perseveration OR spectrum disorder OR savant OR sensory processing disorder AND Immunization OR vaccination OR MMR OR DTAP OR Varicella OR Polio OR Pcv13 AND Autism (("Autistic Disorder/chemically induced"[Majr] OR "Autistic Disorder/etiology"[Majr] OR "Autistic Disorder/immunology"[Majr] OR "Autistic Disorder/microbiology"[Majr] OR "Autistic Disorder/statistics and numerical data"[Majr] OR "Autistic Disorder/virology"[Majr])) OR ("Autistic Disorder/chemically induced"[Mesh:NoExp] OR "Autistic Disorder/etiology"[Mesh:NoExp] OR "Autistic Disorder/immunology"[Mesh:NoExp] OR "Autistic Disorder/microbiology"[Mesh:NoExp] OR "Autistic Disorder/statistics and numerical data"[Mesh:NoExp] OR "Autistic Disorder/virology"[Mesh:NoExp])immunization (("Immunization/adverse effects"[Majr] OR "Immunization/complications"[Majr] OR "Immunization/drug effects"[Majr] OR "Immunization/injuries"[Majr])) OR ("Immunization/adverse effects"[Mesh:NoExp] OR "Immunization/complications"[Mesh:NoExp] OR "Immunization/drug effects"[Mesh:NoExp] OR "Immunization/injuries"[Mesh:NoExp]).
Inclusion criteria
We choose peer-reviewed papers and studies from the last five years written in the English language. We only selected systematic reviews, traditional reviews, meta-analyses, and randomized trials conducted among human subjects. All data collected were within ethical and legal standards.
Exclusion criteria
We excluded gray data and papers that focused on animals. We also excluded articles published before 1998.
Quality assessment tool
We used the following quality assessment tool to evaluate the papers utilized in this study: a Measurement Tool to Assess Systematic Reviews (AMSTAR) questionnaire for systematic reviews and meta-analysis, Cochrane risk bias assessment tools for randomized control trials, the scale for the Assessment of Narrative Review Articles (SANRA) for traditional reviews, and Newcastle-Ottawa Scale for observational studies. We discarded poor-quality studies.
Data collection
We collected the data from the selected articles (with high quality) individually.
Results
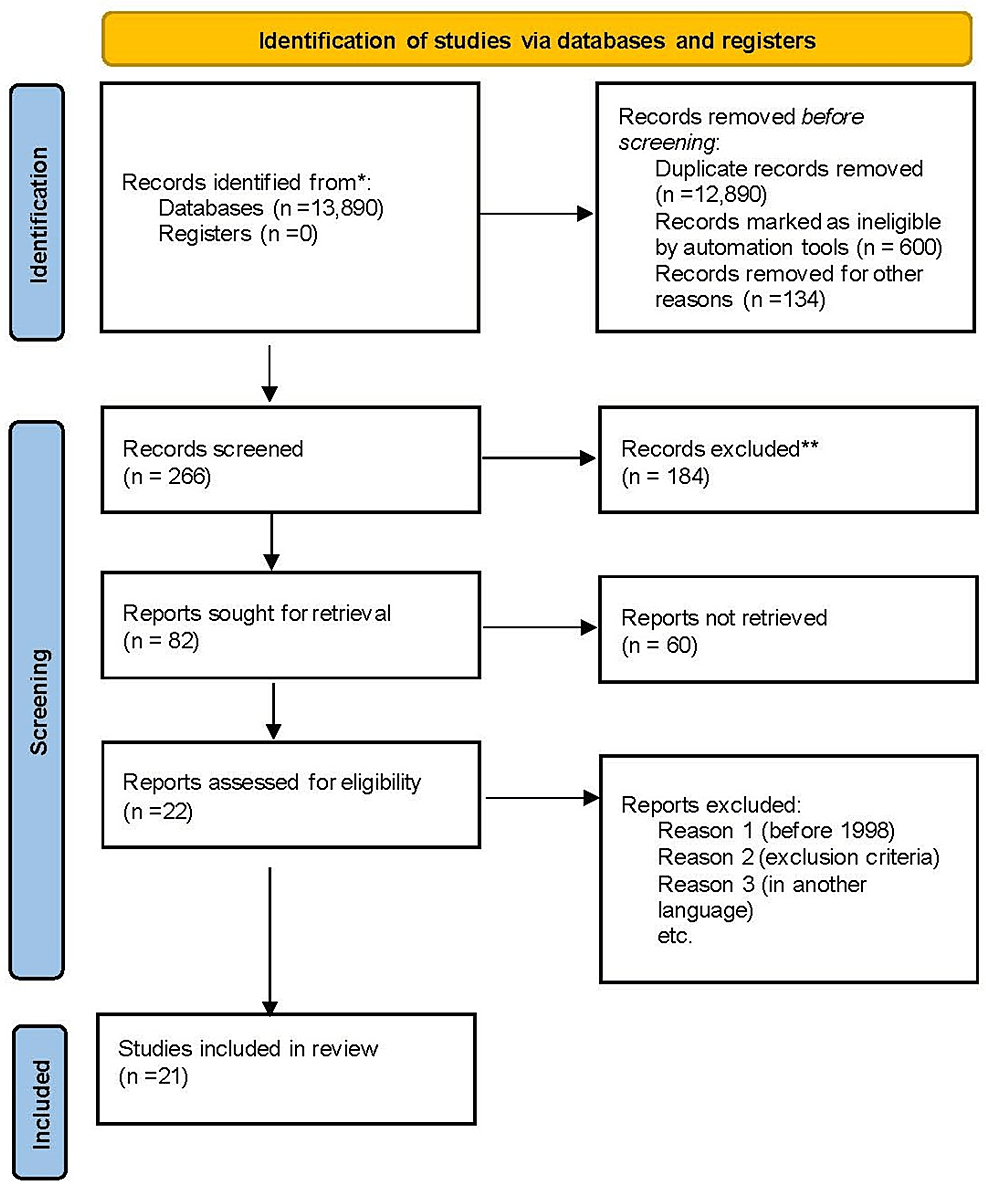
A total of 13,890 records were identified from both Google Scholar and PubMed database (Table 1). We screened 266 records, of which 184 were excluded. After removing duplicates, 600 articles were considered ineligible by automation tools, and 134 were removed for other reasons. We were left with 82 reports for retrieval, 60 were not retrieved, leaving 22 articles. Subsequently, based on the exclusion criteria, we included 21 studies in our study. Figure 1 shows the PRISMA flow diagram.
Table 3 presents the characteristics of the studies included in this systematic review.
Outcomes
In total, 19 articles were on the link between immunization and the incidence of autism. One article discussed the prevalence of ASD, the other about the effect of multiple immunizations during brain development (this study had data supporting the link between the current vaccine schedule and the development of ASD). The results of the 19 articles do not support a causal relationship between childhood immunization and the development of autism.
Discussion
ASD is a collection of phenotypic and developmental disorders resulting in significant social, communicative, and behavioral challenges. It is characterized by speech, language, and social functioning deficiencies and atypical behavioral symptoms, such as habitual, repetitive movements and extreme distress from environmental changes. Comorbidities include mental retardation, epilepsy, chronic gastrointestinal (GI) problems, and hyperactivity in certain persons. Parents of children with ASD frequently notice developmental difficulties during their child's first year of life. The disorder has a vital genetic component [1,5]. Many conditions that were formerly diagnosed as separate entities are now included in ASD diagnosis. These include Asperger syndrome, pervasive developmental disorder (PDD) not otherwise specified, and autistic disorder. ASD with regression is a subtype where patients with ASD have lost previously gained developmental skills, most commonly for language. Unfortunately, there is no cure or single diagnostic approach for the disorder, although some data suggest that early, intensive behavioral therapy may improve functioning [5].
In the late 1990s, Andrew Wakefield, a physician at London's Royal Free Hospital, published a paper in The Lancet claiming to have discovered the measles virus as the cause of autism. Initially, Wakefield stated that the measles virus caused colonic lesions in Crohn's disease. Although this idea was quickly debunked and dismissed, Wakefield was impressed by cases brought to his attention in which typically developing children developed autistic symptoms after receiving the MMR triple vaccine. Despite his prior miscalculation with Crohn's disease, he believed the measles virus had caused inflammatory lesions in the colon. All eight autistic patients on whom he had performed lower GI studies developed the hypothesized lesions, concluding that the measles vaccine virus led to the development of autism [2]. Wakefield's findings could have raised warning lights at this time, even if the measles virus turned out to be a coincidental cause of autism. Wakefield's claim was rapidly followed by reports of the detection of the measles virus in intestinal biopsies, blood, and cerebral spinal fluid samples taken from autistic children. After securing a straightforward and catchy scientific explanation, politicians and leaders of major groups of families of autistic children stood up with Wakefield.
In 2005, an investigative reporter brought to the attention of The Lancet's editors that Wakefield's study had been questioned by severe research misconduct, conflict of interests, and probably mendacity. After an inquiry into the subject, The Lancet withdrew the article, and the British Medical Association took strict actions against Wakefield. Since the Wakefield paper, any attempt to relate autism and the MMR vaccine has been disproved by many studies investigating the epidemiology of autism and the biological effects of MMR and the mumps virus. Mumps viruses were not regularly discovered in autistic children's natural materials at a higher incidence than in non-autistic youngsters. Furthermore, there was no evidence that a decrease in the rate of MMR exposure was associated with reductions in the incidence of autism. Regardless of scientific data, it was assumed that if the mumps virus was not to blame for autism, another MMR ingredient must be [2]. The substantial rise in the incidence of ASD has sparked widespread public concern regarding the disorder's causes and how to prevent them [1]. Despite extensive research about the etiology and pathophysiology of autism, few conclusions have been reached regarding a fundamental causal mechanism. There is no cure currently.
Several hypotheses were posited on the relationship between vaccination and autism development. The first theory relates to immune system dysfunction, organic acid synthesis, the effects of gliamorphin on cerebral function, and mercury toxicity. Parents are concerned about the safety of their children receiving numerous immunizations simultaneously. According to this theory, frequent stimulation of the systemic immune system by vaccination causes a strong microglial reaction in the growing brain, leading to changes in immunological function, resulting in synaptic, dendritic loss, and aberrant appearance pathways. When the microglia are activated, the brain's immune cells release inflammatory cytokines, free radicals, lipid peroxidation products, and two excitotoxins: glutamate and quinolinic acid. Consequently, clinical and pathological characteristics of autism emerge. Microglia are also activated by mercury at quantities of less than 0.5 µg (3 to 5 ng) per gram of moist tissue. High mercuric products are toxic to both the kidneys and the brain. Thimerosal is an organic chemical that includes ethyl mercury and forms a preservative in vaccines. Ethyl mercury hydroxide quickly penetrates the brain and converts to inorganic mercury [6].
Hviid et al. [9] compared children who received immunization with a thimerosal-containing pertussis vaccine to children vaccinated with the same pertussis vaccine formulated without thimerosal and followed them for the symptoms and the signs of autism and other ASD. It was a population-based cohort study to identify the association between thimerosal and autism. In Denmark, they found that the risk of autism and other ASD did not vary greatly between children immunized with a thimerosal-containing vaccine and children immunized with the thimerosal-free vaccine (relative risk (RR) = 0.85 [95% confidence interval (CI) = 0.60-1.20] for autism; RR = 1.12 [95% CI = 0.88-1.43] for other ASD). They also highlighted that there was no proof of a dose-response correlation (increase in RR per 25 µg of ethyl mercury = 0.98 [95% CI = 0.90-1.06] for autism and 1.03 [95% CI = 0.98-1.09] for ASD) [9].
The second hypothesis states that the MMR vaccine can cause autism. Measles is an exceptionally contagious viral infection caused by a paramyxovirus (genus Morbillivirus). It is disseminated through the respiratory system. The clinical features start with a prodrome of flu-like illness, followed by cough, coryza, and conjunctivitis. The measles rash appears as a maculopapular rash on the head that spreads to the torso and arms and legs over three to four days (Koplik spots), which are blue-white plaques on the mucous membranes of the mouth that are pathognomonic. Possible complications include otitis media, viral or bacterial pneumonia, visual loss, acute encephalitis, seizures, and death. Measles is still a significant reason for death and disability in developing countries.
Mumps is a viral infection caused by a paramyxovirus (genus Rubulavirus) transmitted through the respiratory system. Mumps, or measles, virus infection usually results in permanent immunity. Parotitis appears 16-18 days after exposure, and most patients are asymptomatic. Orchitis is more prevalent in post-pubertal boys. Mumps complications are rare and most common in adults, including aseptic meningitis, encephalitis, pancreatitis, and deafness [5].
Rubella (German measles) is another viral infection distributed through the respiratory system. It is caused by togavirus (genus Rubivirus). Fever, malaise, upper respiratory symptoms, and a maculopapular rash are the signs and symptoms of rubella, which appear 14 days after contact. Rubella complications are uncommon, although more common in adults and older children. Congenital rubella syndrome (CRS) is a condition that affects the developing fetus and is more severe when infection occurs early in pregnancy. The rubella virus causes fetal death, early birth, deafness, blindness, and severe birth problems, and infection with the virus usually results in lifetime immunity [5].
MMR Vaccine
The MMR vaccine is part of the required childhood vaccination schedule in the United States. It is given in two doses, the first at 12-15 months and the second at 4-6 years. MMR vaccines are live attenuated virus vaccines to prevent measles (rubeola), mumps, and rubella. It is well-tolerated. The most common side effects are injection site responses, fever (5-15%), and a minor rash (5%). The first shot of the MMR vaccine is associated with afebrile seizures. Thrombocytopenia (low platelet count) is a rare yet severe side effect that happens in roughly 1 in 30,000-40,000 doses. Measles vaccines were available in the United States from the beginning to the mid-1960s, and the MMR vaccine was introduced in 1971. Despite a recurrence of measles in the United States and worldwide from 1989 to 1991, cases continued to drop, and measles was declared eradicated in the United States in 2000.
Mumps, measles, rubella and CRS cases have had a dropdown in the United States since the introduction of the MMR vaccine [5]. Wakefield et al. published a report in 1998 describing 12 cases of pervasive developmental delay and developmental regression linked to GI tract symptoms and developmental regression, many of which occurred shortly after the patient received the MMR vaccine. This case study suggests that a new type of ASD was emerging and linked to the MMR vaccine. Although the study's methodologies were extensively questioned, it raised considerable public worry about the MMR vaccine's safety. Several epidemiological studies have been conducted to understand the association between ASD and the MMR vaccine. These studies have been designed to study multiple hypotheses put forth by the report of Wakefield et al. and others that have advised against using the MMR vaccine. The specific hypotheses that have been studied are (1) rates of ASD are increasing in people who have been given the MMR immunization than in those who have not, (2) a rise in ASD may be occurring as a consequence of the MMR vaccine, (3) the development of ASD is being momentarily linked to receiving the MMR vaccine, and (4) the MMR vaccine may be linked to a new variant form of ASD [4,5].
The tables below will summarize and present the results of these studies; it does not suggest a correlation between ASD and the MMR vaccine [4]. Table 4 shows the characteristic of these studies.
Table 5 shows the comparison of the rate of ASD in vaccinated and unvaccinated Individuals.
Table 6 compares the changes in the rate of ASD with changes in MMR vaccine coverage.
Table 7 shows the temporal association of ASD with the MMR vaccine.
Table 8 shows the specific association between variant autism and the MMR vaccine.
Hviid et al. conducted a nationwide cohort review of all infants born in Denmark to Danish-born mothers from January 1, 1999, through December 31, 2010, to see whether MMR immunization carries a high risk for autism in children, subgroups of children, or periods after vaccination. In Denmark, 657,461 babies born between 1999 and December 31, 2010, participated, with follow-up from one year of age to August 31, 2013 (Danish Civil Registration System is the source of patient information). They found no support for high autism risk after MMR vaccination in a national broad, unselected cohort population of Danish children. In a 2014 meta-analysis of MMR vaccine and autism studies, researchers found two cohorts and four case-control studies from Denmark, Poland, Japan, the United Kingdom, and the United States, with no evidence of a link, for example, a pooled odds ratio from cohort studies of 0.84 (CI = 0.70 to 1.01) [2,4,5,7].
The third hypothesis claims that antenatal Tdap vaccination is linked to a higher risk of ASD. Becerra-Culqui et al. examined the link between antenatal tetanus, diphtheria, and acellular pertussis (Tdap) vaccination and the offspring's risk of ASD. With the rise in the frequency of ASD and the increased vaccination in pregnant women, it is more vital than ever to analyze the safety risks associated with prenatal immunization. This study is a retrospective cohort study of mother-baby pairs that gave birth at Kaiser Permanente Southern California hospitals between January 1, 2011, and December 31, 2014. They used digital medical data to get maternal Tdap immunization from pregnancy to the delivery date. The International Classification of Diseases, Ninth, and Tenth Revision codes conveyed an ASD diagnosis. Children were cared for from birth until their first ASD diagnosis, the end of their membership, or their follow-up (June 30, 2017). According to this study, prenatal Tdap immunization was not linked to an increased incidence of ASD. Table 9 shows the frequencies and associations between Tdap vaccination during pregnancy and ASD in infants born between 2011 and 2014.
Comments
Post a Comment